Unlock the potential of brachytherapy – a game-changing treatment for prostate cancer patients
Alfonso Gomez Iturriaga, MD, PhD from Cruces University Hospital in Bilbao, Spain, explains how this technique stands out from conventional radiation methods, redefining prostate cancer care.

Unprecedented dose precision
Explore why prostate brachytherapy achieves doses previously unattainable with other radiation techniques.
Targeting the Dominant Intraprostatic Lesion (DIL)
Understand how brachytherapy intensifies doses to specific prostate areas while ensuring uniform doses across the rest, minimizing risk to adjacent organs.
Low integral dose to patients
Learn how the internal administration of radiation in brachytherapy minimizes exposure to surrounding healthy tissues, reducing overall radiation impact. Patient selection criteria:
Tumor characteristics
High-risk disease patients benefit most from the dose intensification of brachytherapy.
Patient Factors
Factors like urinary function, IPSS score, and prostate volume guide patient selection, ensuring a positive treatment outcome
Advancing prostate cancer care—A closer look at brachytherapy
Discover a comprehensive exploration of prostate brachytherapy in our article, "Precision and Promise."
Gain insights into the practices at Cruces University Hospital in Bilbao, Spain, where medical experts such as Alfonso Gomez Iturriaga, MD, PhD, and David Büchser MD share valuable perspectives on the precision and patient-centric approaches of prostate brachytherapy. This article offers an informative and educational exploration of brachytherapy's efficacy and patient benefits through real-world case studies and evidence-based discussions. Learn how this therapeutic approach contributes to evolving standards in prostate cancer care. read the full article for in-depth insights into brachytherapy's precision and promise in prostate cancer treatment.
Precision and Promise
Brachytherapy presents many advantages for treating prostate cancer
Prostate cancer is a global health concern, impacting millions of men worldwide. The disease is the most common urological cancer, representing a substantial health burden to the world particularly in developed countries. 1 In 2020, prostate cancer contributed to 14.1 percent and 6.8 percent of all cancer cases and deaths among males. 2 This article presents the advantages of prostate brachytherapy, a modality that has demonstrated a high degree of safety and effectiveness when used as a monotherapy or in conjunction with external beam radiation therapy (EBRT). 3-4
What is prostate brachytherapy?
Brachytherapy is a form of internal radiation therapy designed to target cancer cells directly within affected organs or tissues. A radiation-emitting source is introduced into the body that irradiates and destroys cancer cells. Two types of brachytherapy are used to treat prostate cancer:
Permanent seeds or low dose rate (LDR) brachytherapy.
This form of brachytherapy involves placing small radiation-emitting “seeds” inside the prostate. The seeds emit a specific amount of radiation over a period of months and then cease discharging radiation.
Temporary or high dose rate (HDR) brachytherapy.
HDR is also known as temporary brachytherapy because it delivers a high dose of radiation over a short period of time. A clinician inserts needles into the prostate and then removes the needles, leaving the catheters in place. A radiation-emitting source then moves inside the catheters to a predetermined distance and “dwells” at a location inside the prostate, emitting radiation for five to 15 minutes, after which the source is withdrawn. This provides a concentrated dose of radiation over a short period of time.
Who is eligible for brachytherapy?
Patients diagnosed with prostate cancer may be offered brachytherapy, either LDR or HDR, for several clinical indications:
- Brachytherapy may be used alone for low to favorable intermediate risk prostate cancer patients (intraprostatic disease, Gleason score 6 to 3+4, with low PSA at diagnosis). In this indication, brachytherapy is highly effective in treating prostate cancer. A 2018 study tracked outcomes for 757 people who received brachytherapy for localized prostate cancer between 1990 and 2006. The study found a 17-year prostate specific survival (PsS) rate of 97 percent. 5
- Brachytherapy may be used in combination with EBRT for treating patients presenting with unfavorable intermediate, high and very high-risk prostate cancer (Gleason score 4+3 and higher). According to the American Cancer Society, the addition of brachytherapy to EBRT improves outcomes compared to EBRT alone. Brachytherapy increases the nine-year survival rate from 62 percent to 83 percent for those with immediate to high-risk cancer. 6 The association of EBRT with brachytherapy has shown superior efficacy compared to EBRT alone or even surgery in several recently published series confirming the key role of brachytherapy for these patients. 7-9
- Brachytherapy, especially HDR, may be offered as a salvage intraprostatic treatment for patients relapsing years after a first treatment with EBRT or brachy. Men treated primarily with EBRT or brachytherapy may relapse years after their first treatment, with the disease recurring in the prostate. In this case, if the patient still has a long life expectancy, a salvage treatment may be offered. Surgery, high-intensity ultrasound or cryotherapy are currently proposed in this setting, but results based on a large series of patients have shown that HDR brachytherapy is probably the most reasonable salvage choice because of the lowest risk of side effects. 10-11
Metastatic Advanced Prostate Cancer
Metastatic Advanced Prostate Cancer has spread from the prostate to other parts of the body, most commonly to the bones and lymph nodes. Depending on where the cancer has spread, advanced prostate cancer can cause symptoms, such as fatigue, bone pain, and difficulty urinating. Treatments such as hormones and chemotherapy can help keep the disease under control and to manage symptoms. Patients may be offered EBRT as part of their first treatment. Research has found that combining EBRT with hormone therapy or chemotherapy can help some patients with metastatic prostate cancer live longer. Radiotherapy can also be used to relieve symptoms such as pain in bone metastases. Brachytherapy is generally not used for advanced prostate cancer that has spread to the lymph nodes or distant areas of the body.
The advantages of brachytherapy for prostate cancer
Prostate brachytherapy presents numerous advantages, serving as an effective and patient-centric treatment modality. Excellent long-term results have been published for all risk groups. 12 Physicians have successfully used brachytherapy to treat prostate cancer for many decades. Modern state-of-the-art technologies are used to help healthcare professionals deliver brachytherapy with a high level of precision. Brachytherapy is recognized as a standard treatment alongside surgery and EBRT. 13-14 In high-risk prostate cancer – disease diagnosed in more advanced stages – optimization of local control is critical.
- Clinically proven to be highly effective
15-16
Many studies show that patients remain cancer-free after brachytherapy. Below, we present clinical studies and long-term follow-up data supporting the efficacy and safety of brachytherapy in prostate cancer treatment. - Precision in treatment delivery
By targeting the area where the brachytherapy needles are placed, radiation is given in high doses directly to the prostate with few or no side effects. This precise targeting leads to higher therapeutic ratios, thus maximizing cancer control. - Reduced risk of unnecessary damage to surrounding healthy
tissues and organs
Brachytherapy’s main benefit is limiting radiation exposure to surrounding areas. Healthy structures around the prostate (bladder, rectum, urethra) receive almost no radiation. - Radiation dose customization
The ability to customize radiation doses based on tumor characteristics enables the creation of personalized treatment plans that optimize outcomes while minimizing side effects. - Enhanced patient experience
Patients undergoing brachytherapy often report less pain and discomfort during and after treatment, contributing to a better overall experience. - Minimized side effects
The accurate and targeted nature of LDR and HDR brachytherapy reduces the risk of side effects. 15-16 Avoiding radiation to surrounding critical organs and tissues decreases side effects, thereby contributing to better quality of life. - Shorter treatment times and recovery periods
15-16
Brachytherapy is commonly given on an outpatient basis, encompassing daily treatment sessions during an inpatient stay of a few days. Patients can usually get back to their normal activities within a week. - Minimally invasive
15-16
As a minimally invasive technique, brachytherapy avoids the need for extensive surgery, often making it a desirable alternative to prostatectomy.
Evidence-based outcomes demonstrate the effectiveness of prostate brachytherapy
In this data-driven section, we present compelling clinical studies and long-term follow-up data supporting the efficacy and safety of brachytherapy in prostate cancer treatment.
Several studies show the clinical benefits of HDR boost and salvage
The association of EBRT with brachytherapy has shown superior efficacy compared to EBRT alone or even surgery in several recently published series confirming the key role of brachytherapy for these patients. 7-9
Brachytherapy, especially HDR, may be offered as a salvage intraprostatic treatment for patients relapsing years after a first treatment with EBRT or brachytherapy. Patients treated primarily with EBRT or brachytherapy may relapse years after their first treatment, with the disease recurring in the prostate. In this case, if the patient still has a long life expectancy, a salvage treatment may be offered. Surgery, high-intensity ultrasound or cryotherapy are currently proposed in this setting, but results based on a large series of patients have shown that HDR brachytherapy is probably the most reasonable salvage choice because of the lowest risk of side effects. 10-11
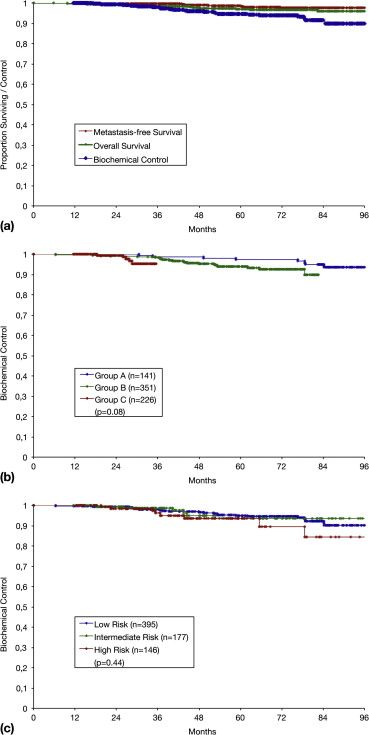
A 718-patient study from Germany reported that HDR brachytherapy
ensures an excellent outcome:
After eight years, biochemical control in prostate cancer was 90
percent, and metastasis-free survival was 97 percent (picture 1).
Biochemical control means that 90 percent of patients were free from
increased PSA (biochemical recurrence) after eight years.
Metastasis-free survival means that 97 percent of patients had no
metastases after eight years.
17
Results of the UK trial comparing external beam radiation therapy (EBRT) alone and EBRT + high dose rate (HDR) brachytherapy shows advantages in relapse-free survival when brachytherapy is added to the treatment: 55% and 71% at six years and 27% and 48% at 12 years. When brachytherapy was added to the treatment, 71% of patients weren’t diagnosed with cancer six years post-treatment, and 48% weren’t diagnosed with experience cancer 12 years post-treatment. 18
The American Brachytherapy Society, with expert input and an extensive literature review, recommends HDR brachytherapy for localized prostate cancer. Research supports this, showing biochemical control rates of 85-100%, 81-100%, and 43-93% for low-, intermediate-, and high-risk cases. HDR offers excellent outcomes with low morbidity, making it a promising primary and salvage treatment choice. 19
Another study highlights excellent outcomes of LDR brachytherapy for intermediate-risk prostate cancer patients, including unfavorable intermediate-risk cases, treated with BED ≥ 200 Gy radiotherapy. 20
Another study looked into the long-term outcome of early-stage prostate cancer treated with brachytherapy after a mean follow-up of seven years. The biochemical recurrence-free survival rate for low-, intermediate- and high-risk patients four to seven years post-brachytherapy ranged from 85%-96%, 74%-97%, and 38%-82%, respectively. 21
Patient and clinical perspectives
Patient insights

Erman
Prostate cancer patient
Brachytherapy gives Erman hope to return to a normal life
Erman, a resident of Basilicata, Italy received some disturbing news from a blood test. His physician told him his PSA (prostate-specific antigen) level was 13.0; the normal range is between 1.0 and 1.5 ng/ml.
“She immediately sent me to the hospital to see a urologist,” says Erman, a married father of two children and two grandchildren. “The doctor who examined me ordered a biopsy of my prostate right away. When the result arrived, my whole world collapsed.”
Erman was diagnosed with prostate cancer and, worse, the surgeon told him he told him he would be unable to surgically remove his prostate, because it was likely he wouldn’t survive the operation.
“You can imagine how I felt,” Erman says. “However, a second doctor – an interventional radiation oncologist – gave me some hope. He would perform a brachytherapy procedure, something I’d never heard of before then.”
After brachytherapy, the physician assured him that everything had gone well.
“I was reborn,” Erman recalls. “A few days after the procedure I already felt better, apart from some difficulty urinating. But mostly I felt relaxed and I was happy to have my normal life back.
“I want to encourage anyone who suffers from the same illness as me to look into brachytherapy, because, in my opinion, it is the least invasive, according to my research on the subject,” he adds. “You will come out of it as happy as I am.”
Clinician insights

Alfonso Gómez Iturriaga, MD, PhD
Radiation Oncologist
Associate Professor of Radiation Oncology
Cruces University Hospital, Bilbao, Spain
Brachytherapy a highly targeted treatment for intermediate to high-risk disease
Physicians at Cruces University Hospital perform two to three HDR brachytherapy procedures each week. HDR brachytherapy and EBRT are preferred in high-risk disease or recurrence cases. Prof. Gómez Iturriaga advocates brachytherapy as a treatment for prostate cancer.
“Brachytherapy excels in delivering high doses of radiation specifically to the prostate while sparing healthy tissues,” he says. “In addition, preserving the prostate – keeping it intact – is crucial for the patient’s quality of life. Brachytherapy provides a high chance of curing the disease while maintaining urinary, bowel, and sexual function.”
In terms of patient selection for HDR brachytherapy, Prof. Gómez Iturriaga explains that it is based on tumor and individual characteristics and generally more beneficial for patients with intermediate to high-risk disease.
“These are the patients that benefit more from the dose intensification associated with brachytherapy,” he says. “Patient characteristics are also quite important – whether the patient can tolerate the treatment. One of these characteristics is the patient’s degree of difficulty in urinating and their IPSS score. In terms of prostate volume, patients with prostates larger than 60 cc’s with poor urinary function may have more side effects than others. Overall, we try to select patients with a high risk of disease and with favorable indicators that brachytherapy would be beneficial.”
Real-time imaging in HDR brachytherapy is a game-changer, Prof. Gómez Iturriaga adds.
“It simplifies the procedure, reduces the time needed, and maintains patient comfort by allowing all steps to be performed in the operating room without moving the patient,” he explains. “We deliver the treatment, remove the needles and the entire procedure is finished in one-and-a-half to two hours.”
Despite the promising outcomes of prostate brachytherapy, Prof. Gómez Iturriaga notes that brachytherapy still is underutilized in Spain. He highlights the need to raise awareness among urologists regarding the procedure's ease and effectiveness.
“Some urologists may hesitate to recommend brachytherapy due to misconceptions about its side effects and difficulty,” he says. “Overcoming this challenge involves educating them about the procedure's modern, efficient, and low-risk nature.”
Prof. Gómez Iturriaga adds that setting up a prostate brachytherapy program is relatively straightforward if the institution already conducts other forms of brachytherapy. It requires appropriate software, ultrasound equipment, and training. Observerships in experienced centers can help those new to brachytherapy get started.
He stressed that the accuracy, conformity, and clinical data supporting brachytherapy make it a compelling choice. The future may involve combining new imaging techniques with the precision of brachytherapy, high radiation doses, and brachytherapy combined with SBRT.

David Büchser, MD
Radiation oncologist
Cruces University Hospital, Bilbao, Spain
Delivering dose to the disease, avoiding healthy tissues a hallmark of brachytherapy
David Büchser, MD, who with his team performs two to three prostate brachytherapy procedures weekly, highlights prostate brachytherapy's precision, effectiveness, and patient-centered approach, emphasizing its value in treating localized prostate cancer.
“Prostate brachytherapy is the radiation technique that is the most precise by far,” he says. “It allows extreme dose escalation within the prostate while preserving the surrounding healthy tissues from high radiation doses. That means you maximize the chances of tumor control while reducing the rates of acute and late toxicities. Therefore, many patients with localized prostate cancer can benefit from LDR or HDR prostate brachytherapy.”
David Büchser, MD, emphasizes real-time imaging's significance, as it allows the procedure to be performed with the patient in position, improving treatment reliability.
“Without real-time imaging, after you perform the implant you would need to reposition the patient’s legs to transport him out of the OR and to the CT or MR suite for imaging, then return the patient to the treatment room to complete the whole dosimetry,” he says. “All of this replacing and moving the patient adds uncertainty to the process. Real-time imaging eliminates those uncertainties and allows you to perform the whole procedure with the patient in position and without movement, resulting in a very solid protocol.”
When establishing a real-time HDR program, it is critical that clinicians be well-versed in transrectal ultrasound and have a solid understanding of HDR prostate brachytherapy dosimetry, according to David Büchser, MD.
“If a center has no experience in prostate brachytherapy, a staff member with experience in transrectal ultrasound and the placement of needles through the peritoneum would be very helpful,” he observes.
He encourages peers to consider starting a prostate brachytherapy program, especially those already using external beam radiation therapy for prostate cancer.
“Prostate brachytherapy allows for dose escalation within the prostate, thereby enhancing disease control without compromising patient functionality,” David Büchser, MD, says.
David Büchser, MD, recognizes the ongoing challenge of raising awareness among colleagues about the value of prostate brachytherapy.
“Despite the mountain of evidence of the benefit of prostate brachytherapy, we will have to strive to make clinicians see that the modality plays a key role in the management of patients with localized prostate cancer,” he says. “While external beam radiotherapy developments may seem appealing, we have to make an effort to demonstrate how prostate brachytherapy can help us achieve better outcomes, while preserving patient functionality, making it an attractive treatment option.”

Juan Carlos Moscarei, MD
Radiation oncologist
Cruces University Hospital, Bilbao, Spain
As a professional discipline, brachytherapy offers clinicians a rich career experience
In exploring medical specialties during his training, Dr. Moscarei saw radiation oncology as a discipline that would offer a wide range of opportunities.
“It had everything I was looking for in a specialty,” he says. “It is a decisive treatment modality and the daily work can be quite varied – there’s the clinic, there’s contouring and imaging, and, above all, the chance to have patient contact. Plus, brachytherapy is an ‘artisan technique’ that depends on skill. All of this seemed very attractive to me so I decided I would like to practice brachytherapy.”
Dr. Moscarei derives the most satisfaction from the opportunity to help patients in the best possible way.
“The benefit that brachytherapy brings to patients is mainly dosimetry,” he explains. “This allows dose escalation without increasing toxicity to organs-at-risk, in turn, enabling more curative treatments or therapy that is less toxic or with shorter treatment times.
“To new physicians who are undecided,” Dr. Moscarei continues, “I would say that brachytherapy is a technique that requires a desirable level of craftsmanship and one that enables more complete and greater diversity of treatments.”
Although he has been training for only a short time, he still remembers the first case in which he introduced the brachytherapy needles.
“I recall that when I saw him 48 hours later in consultation, the patient was asymptomatic and maintaining his quality of life and that gave me a lot of satisfaction,” he says. “Subsequently, he is doing well and that also makes me very happy with the treatment.”

José María Espinosa, PhD
Medical physicist
Cruces University Hospital, Bilbao, Spain
Real-time ultrasound imaging reduces uncertainty during brachytherapy
In planning prostate brachytherapy, there is no substitution for real-time imaging, according to Dr. Espinosa, who has been using ultrasound with the patient in the treatment position.
“We believe that it is a fundamental advantage to use real-time ultrasound to reduce geometric uncertainty,” he says. “We first carry out a pre-plan using ultrasound, so that we will be able to best decide where to put the brachytherapy needles – repositioning them if necessary and thus obtaining a treatment appropriate to the patient’s geometry.”
The next evolution of the prostate brachytherapy Dr. Espinosa’s team has been performing for the last 10 years will be the elimination of manual steps throughout the entire treatment, he predicts.
“In particular, if we could implement a system that identifies all the treatment tubes – just as it exists in gynecological brachytherapy in which the first three tubes are identified – we would not have any manual errors in tube placement,” Dr. Espinosa explains. “If an electronic system connected to the software could be installed in the needle insertion grid to inform you about which needle to select we believe that manual errors could be avoided.”
He adds that an acceleration of the speed of the Monte Carlo dose calculation – which would account for tissue inhomogeneities or the presence of air – and an improvement of the fusion algorithms to help ascertain focal prostate areas that could receive a higher dose, could also bring prostate brachytherapy to the next level.
Conclusion
Brachytherapy excels in precision, effectiveness, and patient-centered care for prostate cancer treatment. It precisely targets prostate tumors, maximizing cancer control while minimizing side effects through tailored radiation doses. Patients experience minimal pain and discomfort, and benefit from shorter treatment times, faster recovery, and fewer side effects.
Although brachytherapy’s adoption worldwide is still relatively low – necessitating increased awareness and education among radiation oncologists and urologist – the modality offers hope to prostate cancer patients by virtue of its many benefits. Clinical evidence consistently demonstrates its efficacy and safety, with high long-term success rates. 22-25
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics
2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide
for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021
May;71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4.
PMID: 33538338.
2. https://gco.iarc.fr/today/home
3. Behmueller M, Tselis N, Zamboglou N, et al. High-dose-rate
brachytherapy as monotherapy for low- and intermediate-risk
prostate cancer. Oncological outcomes after a median 15-year
follow-up. Front Oncol. 2021 Dec 2;11:770959. doi:
10.3389/fonc.2021.770959. PMID: 34926278; PMCID: PMC8674679.
4. Hjälm-Eriksson M, Nilsson S, Brandberg Y, et. al. High rate
of local control and cure at 10 years after treatment of
prostate cancer with external beam radiotherapy and
high-dose-rate brachytherapy: a single centre experience. Acta
Oncol. 2021 Oct;60(10):1301-1307. doi:
10.1080/0284186X.2021.1953706. Epub 2021 Sep 9. PMID:
34498986.
5. Lazarev S, Thompson MR, Stone NN, et al. Low-dose-rate
brachytherapy for prostate cancer: outcomes at >10 years of
follow-up. BJU Int. 2018 May;121(5):781-790. doi:
10.1111/bju.14122 . Epub 2018 Jan 30. PMID: 29319928.
6. Rodda S, Morris WJ, Hamm J, et al. ASCENDE-RT: An analysis of
health-related quality of life for a randomized trial comparing
low-dose-rate brachytherapy boost with dose-escalated external
beam boost for high- and intermediate-risk prostate cancer. Int
J Radiat Oncol Biol Phys. 2017;98(3)581-589.
7. Kishan AU, Cook RR, Ciezki JP, et al. Radical prostatectomy,
external beam radiotherapy, or external beam radiotherapy with
brachytherapy boost and disease progression and mortality in
patients with Gleason score 9-10 prostate cancer. JAMA. 2018 Mar
6;319(9):896-905. doi: 10.1001/jama.2018.0587. PMID: 29509865;
PMCID: PMC5885899.
8. Tilki D, Chen MH, Wu J, et al. Surgery vs radiotherapy in the
management of biopsy Gleason score 9-10 prostate cancer and the
risk of mortality. JAMA Oncol. 2019 Feb 1;5(2):213-220. doi:
10.1001/jamaoncol.2018.4836. PMID: 30452521; PMCID:
PMC6439553.
9. Kishan AU, Karnes RJ, Romero T, et al. Comparison of
multimodal therapies and outcomes among patients with high-risk
prostate cancer with adverse clinicopathologic features. JAMA
Netw Open. 2021 Jul 1;4(7):e2115312. doi:
10.1001/jamanetworkopen.2021.15312. PMID: 34196715; PMCID:
PMC8251338.
10. Valle LF, Lehrer EJ, Markovic D, et al. A systematic review
and meta-analysis of local salvage therapies after radiotherapy
for prostate cancer (MASTER). Eur Urol. 2021 Sep;80(3):280-292.
doi: 10.1016/j.eururo.2020.11.010. Epub 2020 Dec 11. PMID:
33309278; PMCID: PMC10262981.
11. Kissel M, Pounou A, Ka K, Alexis A, et al. Efficacy and
toxicity following salvage high-dose-rate brachytherapy for
locally recurrent prostate cancer after radiotherapy.
Brachytherapy. 2022 Jul-Aug;21(4):424-434. doi:
10.1016/j.brachy.2022.01.005. Epub 2022 Mar 21. PMID:
35331666.
12. American Brachytherapy Society. Prostate Brachytherapy –
LDR.
https://www.americanbrachytherapy.org/resources/for-professionals/current-applications-of-brachytherapy/prostate-brachytherapy-ldr/#:~:text=Prostate%20Brachytherapy%20%2D%20Indications&text=For%20most%20men%20with%20high,9%2D12%20months%20is%20appropriate.
13. Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG
Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and
Local Treatment with Curative Intent. European Urology, Volume
71, Issue 4, 2017, Pages 618-629, ISSN 0302-2838,
https://doi.org/10.1016/j.eururo.2016.08.003.
14. Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG
Guidelines on Prostate Cancer. Part II: Treatment of Relapsing,
Metastatic, and Castration-Resistant Prostate Cancer. European
Urology, Volume 71, Issue 4, 2017, Pages 630-642, ISSN
0302-2838, https://doi.org/10.1016/j.eururo.2016.08.002.
15. Grimm P, Billiet I, Bostwick D, et al. Comparative analysis
of prostate-specific antigen free survival outcomes for patients
with low, intermediate and high risk prostate cancer treatment
by radical therapy. Results from the Prostate Cancer Results
Study Group. BJU Int. 2012 Feb;109 Suppl 1:22-9. doi:
10.1111/j.1464-410X.2011.10827.x. PMID: 22239226.
16. Morton GC at al. Clin Oncol. 2020;32(3):163-9.
https://www.clinicaloncologyonline.net/article/S09366555(19)30491-1/fulltext
17. Zamboglou N, Tselis N, Baltas D, et al. High-dose-rate
interstitial brachytherapy as monotherapy for clinically
localized prostate cancer: treatment evolution and mature
results. Int J Radiat Oncol Biol Phys. 2013 Mar 1;85(3):672-8.
doi: 10.1016/j.ijrobp.2012.07.004. Epub 2012 Aug 25. PMID:
22929859.
18. Hoskin, PJ, Rojas, AM, Ostler, PJ, et al. Randomised trial
of external-beam radiotherapy alone or with high-dose-rate
brachytherapy for prostate cancer: Mature 12-year results,
Radiotherapy and Oncology, Volume 154, 2021, Pages 214-219, ISSN
0167-8140, https://doi.org/10.1016/j.radonc.2020.09.047.
19. Yamada Y, Rogers L, Demanes DJ, et al. American
Brachytherapy Society consensus guidelines for high-dose-rate
prostate brachytherapy. Brachytherapy. 2012 Jan-Feb;11(1):20-32.
doi: 10.1016/j.brachy.2011.09.008. PMID: 22265435.
20. Okamoto K, Okuyama K, Kohno N, et al. Clinical outcomes of
low-dose-rate brachytherapy based radiotherapy for intermediate
risk prostate cancer. J Contemp Brachytherapy. 2020
Feb;12(1):6-11. doi: 10.5114/jcb.2020.92405. Epub 2020 Feb 28.
PMID: 32190064; PMCID: PMC7073334.
21. Yan W, Chen J, Zhou Y, et al. Long-term outcome of early
stage prostate cancer treated with brachytherapy analysis after
a mean follow-up of 7 years. Springerplus. 2014 Jul 15;3:357.
doi: 10.1186/2193-1801-3-357. PMID: 25089248; PMCID:
PMC4117862.
22. Wegener E, Samuels J, Sidhom M, et al. Virtual HDR boost for
prostate cancer: rebooting a classic treatment using modern
tech. Cancers (Basel). 2023 Mar 28;15(7):2018. doi:
10.3390/cancers15072018. PMID: 37046680; PMCID: PMC10093761.
23. Crook, J Marbán M, Batchelar, D. HDR Prostate Brachytherapy,
Seminars in Radiation Oncology, Volume 30, Issue 1,
24. 2020, Pages 49-60, ISSN 1053-4296,
https://doi.org/10.1016/j.semradonc.2019.08.003.
(https://www.sciencedirect.com/science/article/pii/S1053429619300578)
25. Zhang H, Kang S, Ali N, et al. Building a high-dose-rate
prostate brachytherapy program with real-time ultrasound-based
planning: Initial safety, quality, and outcome results. Open
Access: Published: February 21, 2020. DOI:
https://doi.org/10.1016/j.adro.2020.02.002.
How brachytherapy transforms prostate cancer treatment
Watch exclusive videos. Click below to explore.

3 key advantages
Brachytherapy over other radiation treatments and discusses patient selection criteria.
Ready to implement Prostate Brachytherapy in your practice?
Book a meeting with our specialists to help you get started.
Brachytherapy linked to major decrease in radical prostatectomy for inroads for prostate cancer
Discover key insights from Gerard Morton, MD, on managing prostate cancer
Brachytherapy linked to major decrease in radical prostatectomy for inroads for prostate cancer
While HDR brachytherapy use still lags in Canada, clinical evidence is driving reliance on the modality as monotherapy or boost
Although due to several factors, HDR brachytherapy is used in only three percent of prostate cancer cases in Canada, its utilization for high-risk and unfavorable intermediate risk prostate cancer is increasing as clinical evidence mounts regarding the therapy’s efficacy, safety, side effect profile and precision.
“In Canada, there is a huge amount of regional variation in brachytherapy’s use,” says Gerard Morton, MD, a radiation oncologist at Odette Cancer Centre (Sunnybrook Health Sciences Centre) and professor of radiation oncology at the University of Toronto (Ontario, Canada). “This is due to physician preference, physician training, clinicians’ biases either for or against brachytherapy, as well as geographic differences in reimbursement.”
In Ontario – where Dr. Morton has been performing HDR brachytherapy since 2001 – however, the modality’s use as a boost after EBRT rose during the period 2006 to 2017. 1
“There was a significant increased utilization in prostate brachytherapy, particularly as a boost for higher risk disease,” Dr. Morton says. “The number of patients who were treated by brachytherapy as a monotherapy remained fairly steady, however there was a big difference seen in the management of higher risk disease. HDR boost was used much more frequently, and accompanied by a major decrease – from 58 percent in 2007 to 45 percent in 2017 – in men having radical prostatectomies, while at the same time brachy boost increased from 1.6 percent to 10.4 percent in that same decade.” 1
“It was interesting and comforting to see this increased utilization of brachytherapy,” says Gerard Morton, MD, a radiation oncologist at Odette Cancer Centre (Sunnybrook Health Sciences Centre) and professor of radiation oncology at the University of Toronto (Ontario, Canada). “A big driver for that was likely the 2017 evidence-based guidelines by Cancer Care Ontario and ASCO, which strongly recommend the use of brachytherapy boost – either HDR or LDR – for men with high risk or unfavorable intermediate risk prostate cancer.” 2
Contemporaneous studies also support the clinical value of HDR brachytherapy as a boost before or after EBRT 3-5 ; for salvage in cases of intraprostatic recurrence after EBRT or LDR brachytherapy 6-7 ; and for minimized side effects 8 (e.g., erectile/urinary function.)
While even within Ontario, brachytherapy utilization can range widely, as low as five percent in some centers to over 50 percent in others – attributable to the aforementioned clinician-related factors – Odette Cancer Centre has adopted HDR brachytherapy as its standard approach for patients with higher risk disease.
“We found that the biggest predictor of brachytherapy use was the physician the patient saw.”
“We found that the biggest predictor of brachytherapy use was the physician the patient saw,” Dr. Morton observes. “It really ties in with biases and physician preference versus anything else.”
Decision-making on therapy a multi-disciplinary pursuit
Historically, the urologist has been the “gatekeeper” concerning which treatment a patient with high-risk prostate cancer should receive. As urologists generally are not involved in brachytherapy delivery, prostatectomy was prescribed more often than EBRT or brachytherapy, the latter options viewed by many urologists as risking undesirable side effects, he notes.
“Here at Odette and many other institutions there is a mandate to provide quality care for men who have been diagnosed with prostate cancer, and quality of care requires a multi-disciplinary [MDT] assessment,” Dr. Morton says. “In the MDT assessment, I and my urologist colleagues systematically see every patient diagnosed with prostate cancer.
“It turns out that 70 percent of patients who have an MDT assessment opt for some form of radiation rather than radical prostatectomy.”
“It turns out that 70 percent of patients who have an MDT assessment opt for some form of radiation rather than radical prostatectomy,” he continues. “That gives us an opportunity to explore for individual patients what the optimal form of radiation might be. For many it’s brachytherapy, for many it’s not. For instance, the patient may have comorbidities or a large prostate or poor urinary function that contraindicates that approach. So, not everyone should have brachytherapy, but it should certainly be offered to many patients.”
The insight of clinicians isn’t the patient’s only resource within the MDT clinic; patients can also consult with a volunteer from a patient support network, members of which have had prostate cancer themselves and are well-versed and knowledgeable from a lay perspective in prostate cancer treatment.
“They’re independent of the physician, and patients love having the opportunity to speak to patient peers, because the patient’s voice often is viewed as more trustworthy and less biased than that of the clinician,” Dr. Morton explains. “Patients really appreciate the ability to interact with those who have been through the various treatments and who can report on their personal outcomes of surgery, EBRT and brachytherapy.”
HDR boost and monotherapy patients
Most of the cases that Dr. Morton treat with HDR boost are high risk patients, especially those with bulky disease, good urinary function, prostates that aren’t too large and no comorbidities that would prevent the use of anesthesia. These criteria make them ideal candidates for EBRT + boost HDR brachytherapy.
Patients who qualify for HDR monotherapy are those with favorable intermediate risk prostate cancer. Dr. Morton recently consulted on treatment options with three men, aged 61-, 52- and 50-years-old, all with Grade Group 2 disease.
“Generally, we would recommend – certainly in North America – that they have treatment rather than surveillance,” he says. “When the patient looks at the morbidity of different treatment approaches, brachytherapy really stands out as having a lot to offer them. We could recommend SBRT, but I do have concerns about the long-term consequences of a big dose of external beam radiation for a 50-year-old, since that individual can expect to live probably another 30 years. There is a significant potential for a second malignancy over that period of time, or the unknown consequences of delivering a very large dose per fraction with EBRT.”
Rather than SBRT, Dr. Morton would offer a prostatectomy, and accept the possible extra morbidity, or brachytherapy.
“Many of these patients prefer to have brachytherapy over surgery,” he says. “These three patients were perfect cases for HDR monotherapy.”
HDR workflow
In 2001, when Odette Cancer Centre established its HDR brachytherapy service, it used CT-based planning, then, in 2009, migrated to real-time ultrasound-based planning with Oncentra Prostate, which Dr. Morton says is “a wonderful way to deliver prostate HDR.”
On treatment day, the patient enters the operating room and is placed under general anesthesia. Needle implantation is performed using a standardized template, followed by imaging, plan development and QA checks. The treatment is then delivered with the patient still anesthetized, after which the catheters are withdrawn and the patient is awakened.
“Typically, brachytherapy takes 60 to 90 minutes from start to finish, so it’s a very efficient and accurate way to deliver treatment.”
“Typically, brachytherapy takes 60 to 90 minutes from start to finish, so it’s a very efficient and accurate way to deliver treatment,” he says. “Unlike when we did CT-based planning, we don’t have to worry about catheter displacement, as the patient is under general anesthesia for the whole procedure and the ultrasound probe is in place the entire time as well.”
Brachytherapy’s future for prostate cancer
Ongoing and future studies on the clinical value of brachytherapy for prostate cancer will move the needle on the modality’s use, according to Dr. Morton.
“What we need is really solid evidence that brachytherapy is best – that will drive practice in the future,” he says. “Right now, despite positive studies coming out every year, brachytherapy is still subject to various perceptions and misperceptions – perhaps about toxicity or maybe greater expectations about competing modalities, especially SBRT.
“What we need is a good randomized clinical trial,” Dr. Morton adds, “and I’m happy to note that we have a Canadian clinical trial opening at the beginning of 2024 that has been approved in the United States by CTEP. It’s going to be open to many US centers as well, which will be randomizing men who have high risk prostate cancer and intermediate risk prostate cancer to either brachytherapy boost or five-fraction SBRT, because I think this is going to be the question people will be asking in the future. We need to show that brachytherapy is non-inferior to SBRT, so to do that we need to do these clinical trials.”
References
Insights into prostate cancer brachytherapy
Perspectives from Alberto Bossi, MD, an radiation oncologist
Invaluable insights into prostate cancer brachytherapy across enlightening videos.
Enhancing local control and minimizing side effects through brachytherapy
By utilizing brachytherapy as a boost to external beam radiotherapy, higher doses can be delivered to the prostate, enhancing local disease control. Moreover, combining brachytherapy with other treatments, especially HDR brachytherapy, minimizes side effects, offering patients a more favorable toxicity profile.
Prioritizing patient well-being in prostate cancer treatment
Understanding their concerns about surgery, radiotherapy, or brachytherapy side effects is crucial for personalized treatment decisions. He advocates for thorough discussions, not just about oncological outcomes but also about potential complications. Ultimately, informed patients can make choices that align with their future quality of life.
Optimal start with real-time brachytherapy
Alberto Bossi, MD, recommends real-time procedures for initiating brachytherapy. With immediate control and dosimetry on echography images, it ensures efficiency, which is crucial for treating multiple patients daily. Familiarity with echography imaging enhances the process, making real-time procedures the preferred choice for starting brachytherapy.
Ready to implement Prostate Brachytherapy in your practice?
Book a meeting with our specialists to help you get started.
Revolutionizing HDR Brachytherapy Workflow with Real-Time Integration
Experience Seamless Treatment from Imaging to Delivery with Oncentra® Prostate.
Learn how Elekta’s Oncentra Prostate solution revolutionizes HDR brachytherapy by allowing the entire process to unfold within the procedure room. This article unveils the streamlined, safe, and efficient workflow enabled by Oncentra Prostate, from initial imaging to treatment delivery.
Key Features:
- Complete HDR brachytherapy process within the procedure room
- Ultrasound-guided, real-time HDR workflow ensures safety and precision.
Transformative Approach:
Experience a shift from conventional methods to a seamless, efficient workflow for HDR brachytherapy in prostate cancer treatment.
Step-by-Step Guide:
Explore the process of equipment and patient preparation, virtual planning, needle insertion, reconstruction, and treatment delivery.
Integrated Solution:
Oncentra Prostate stands as a fully dedicated solution for HDR brachytherapy. Developed to provide a real-time workflow, it integrates all components seamlessly to ensure highly conformal and uniform treatment plans, meeting clinical objectives with efficiency and precision.
A streamlined HDR brachytherapy workflow for prostate cancer
Elekta’s Oncentra Prostate offers an ultrasound-guided, real-time HDR workflow, enabling a safe and controlled procedure
Prostate cancer is the most common urological cancer particularly in developed countries. 1 Fortunately, prostate cancer is eminently treatable with surgery, EBRT – with or without HDR brachytherapy boost – or with HDR brachytherapy alone. Due to its ability to precisely target prostate cancer from inside the gland, as well as its safety and efficacy, HDR brachytherapy, either by itself or in conjunction with EBRT, has proven to be an attractive technique, especially for intermediate- and high-risk disease. 2-17
Following ultrasound-guided needle insertion, the conventional HDR brachytherapy workflow requires the patient to be transported to the CT suite for imaging to confirm the accuracy of implanted needle positions, then transported again after treatment planning, to the procedure room for treatment delivery. This patient movement risks needle displacement and results in a time-consuming workflow.
Conversely, the real-time Oncentra® Prostate solution provides a way to complete the entire HDR brachytherapy process in the procedure room, including initial ultrasound imaging, contouring, ultrasound-guided needle insertion, reconstruction, dose planning, and treatment delivery. Most important, this streamlined workflow enables physicians to plan needle paths virtually – using ultrasound images – before needle insertion. After needle insertion, it is possible to modify the positions of inserted needles and to further optimize the plan before treatment delivery. This leads to a reduced risk of needle displacement and streamlines the workflow, thus saving time.
Real-time Oncentra® Prostate HDR brachytherapy
The equipment needed to perform Oncentra Prostate HDR brachytherapy includes:
- Cart-mounted Oncentra Prostate planning system dedicated for HDR/LDR prostate brachytherapy
- Ultrasound system and probe (user-selected)
- Endo-cavity Rotational Mover (ECRM) with probe cradle
- OncoSelect stepper adapter
- Prostate template and stepper holder
- Flexitron or microSelectron afterloader
Equipment preparation
Before the patient enters the procedure room the user powers on the ultrasound system, connects the ultrasound probe, and checks the video signal on Oncentra Prostate. The user also connects the stepper-encoder on Oncentra Prostate and confirms its functionality, in addition to the functionality of the virtual ultrasound template (i.e., pattern recognition).
Then, the ultrasound probe is mounted vertically on the stepper and the brachy balloon over the probe is filled with water. Next, the user mounts and fixes the prostate template onto the stepper.
Patient preparation
Before or after the patient enters the procedure room, the treatment area is shaved. In the treatment room he is positioned on the patient table, with optimal leg support and a sterile cover, and the treatment area is disinfected. In accordance with patient desires and clinical requirements, the patient may subsequently receive local or general anesthesia. A bladder catheter is inserted to provide a contrast agent to visualize the urethra. At some point during patient preparation, the user inputs administrative patient data in Oncentra Prostate.
Before virtual planning
Acquiring clear images of the prostate is important to ensure the success of virtual planning. The ultrasound probe on the stepper adapter is inserted into the rectum and freehand scanning of the prostate is performed. The middle position of the stepper should provide a high quality image of the urethra in the sagittal (i.e., longitudinal) plane. For sagittal imaging, the ECRM enables automated rotational (sweeping) movement of the ultrasound probe and both are directly controlled by Oncentra Prostate.
The user confirms that the image quality on both axial and sagittal planes is acceptable and, if needed, optimizes depth, focus and contrast. Subsequently, the virtual template is displayed on the ultrasound image.
Next, the actual template (e.g., Martinez Prostate Template) on the stepper holder is advanced toward the patient until it comes into contact with the patient’s perineum and the stepper is covered with a sterile drape. At this stage, to prevent misalignment of the prostate and template during needle insertion, the user may optionally insert an anchor needle to fix the prostate to the template.
To reference the stepper position toward the anatomical landmark of
the base plane (i.e., base of prostate), appearing on the axial
plane, is designated as stepper origin with position 0.
Now, a 3D ultrasound sequence of the prostate region will be
acquired either by motorized rotational acquisition (with ECRM) or
continuous movement in translational direction of the US probe from
base towards apex.
Virtual planning
Before the user contours the target and OARs (rectum, urethra), axial and sagittal live 3D ultrasound imaging is used to check image quality, and to confirm the base plane, reference plane and apex distances to the template, as well as prostate volume. Because Oncentra Prostate employs protocol-based planning, the user can load all relevant preferences and presets, including treatment aim, the number of needles to be used, the distance of the needles to the prostate, the colors of the isodoses and needles, in addition to the contour colors for the target and OARs.
Advanced contouring tools make contouring of the prostate and OARs simple, precise and fast. These tools include Envelope (prostate), Sweep (urethra) and Pearl (rectum).
Next, a virtual treatment plan based on the user’s dosimetry objectives is proposed by Oncentra Prostate’s Hybrid Inverse Planning & Optimization (HIPO) algorithm. In about 30 seconds, HIPO – using presets (e.g., dose constraints, weights) for each organ – offers a plan that specifies needle positions, dwell times and dwell positions.
Users can then evaluate virtual needle positions relative to OARs and the prostate, and move, adjust or delete needles manually. Users also check the DVHs for conformity and homogeneity. When all criteria are met, the user prints the protocol for loading the template with actual needles.
Needle insertion
With the planned needle trajectories loaded into Oncentra Prostate, the user is ready to insert needles into the template. Starting with ventral needles, a needle is selected on the virtual display and the ultrasound probe will automatically rotate (via ECRM) to the correct plane on the live sagittal ultrasound image. Users can watch in real time as they advance the needle through the template and into the prostate, employing the sagittal image to insert the needle to the right depth (i.e., apex to base). Once a needle is in place, the user reconstructs the precise needle position and updates the plan.
Reconstruction
Because multiple needle insertions can deform the prostate’s shape, Oncentra Prostate enables the user to adapt needle positions in real time and reconstruct target volumes for recontouring, dose recalculation and evaluation. Users can capture an up-to-date image set at any time, enabling simple plan adjustments to the in vivo situation. This is accomplished through Dynamic Needle Tracking, which monitors the implanted needle configuration and calculates deviations between planned and actual needle positions. “Traffic light” indicators provide quick visual insight about the level of alignment of the presets with the planned dosimetry.
Treatment delivery
Once the optimized plan is imported from Oncentra Prostate to the afterloader, the user checks the treatment channel loading and dwell times. Then transfer tubes are connected; left top is first catheter and right bottom is last catheter, making sure not to manipulate needle depths. Users then leave the treatment room and enter the control room, where they can monitor the patient and anesthesia equipment. Dose delivery can now begin.
After treatment delivery, anchor needles and treatment needles are withdrawn, in addition to the ultrasound probe and template. Caregivers address any patient needs, such as pressure bandages or bladder catheter, and the patient leaves the procedure room.
The final step is cleaning of the ultrasound probe, disinfection of the stepper and sterilization of the template.
A truly integrated, fully dedicated solution for HDR brachytherapy
Oncentra Prostate was developed to offer a real-time workflow for HDR prostate brachytherapy. The integration of all components in a single solution ensures that products interact seamlessly. With a design that results in highly conformal and uniform treatment plans, users can be sure that clinical objectives are met in the most efficient and dedicated way.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338.
- Behmueller M, Tselis N, Zamboglou N, et al. High-dose-rate brachytherapy as monotherapy for low- and intermediate-risk prostate cancer. Oncological outcomes after a median 15-year follow-up. Front Oncol. 2021 Dec 2;11:770959. doi: 10.3389/fonc.2021.770959. PMID: 34926278; PMCID: PMC8674679.
- Hjälm-Eriksson M, Nilsson S, Brandberg Y, et. al. High rate of local control and cure at 10 years after treatment of prostate cancer with external beam radiotherapy and high-dose-rate brachytherapy: a single centre experience. Acta Oncol. 2021 Oct;60(10):1301-1307. doi: 10.1080/0284186X.2021.1953706. Epub 2021 Sep 9. PMID: 34498986.
- Kishan AU, Cook RR, Ciezki JP, et al. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with Gleason score 9-10 prostate cancer. JAMA. 2018 Mar 6;319(9):896-905. doi: 10.1001/jama.2018.0587. PMID: 29509865; PMCID: PMC5885899.
- Tilki D, Chen MH, Wu J, et al. Surgery vs radiotherapy in the management of biopsy Gleason score 9-10 prostate cancer and the risk of mortality. JAMA Oncol. 2019 Feb 1;5(2):213-220. doi: 10.1001/jamaoncol.2018.4836. PMID: 30452521; PMCID: PMC6439553.
- Kishan AU, Karnes RJ, Romero T, et al. Comparison of multimodal therapies and outcomes among patients with high-risk prostate cancer with adverse clinicopathologic features. JAMA Netw Open. 2021 Jul 1;4(7):e2115312. doi: 10.1001/jamanetworkopen.2021.15312. PMID: 34196715; PMCID: PMC8251338.
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. European Urology, Volume 71, Issue 4, 2017, Pages 618-629, ISSN 0302-2838, https://doi.org/10.1016/j.eururo.2016.08.003.
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. European Urology, Volume 71, Issue 4, 2017, Pages 630-642, ISSN 0302-2838, https://doi.org/10.1016/j.eururo.2016.08.002.
- Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012 Feb;109 Suppl 1:22-9. doi: 10.1111/j.1464-410X.2011.10827.x. PMID: 22239226.
- Morton GC at al. Clin Oncol. 2020;32(3):163-9. https://www.clinicaloncologyonline.net/article/S09366555(19)30491-1/fulltext
- Valle LF, Lehrer EJ, Markovic D, et al. A systematic review and meta-analysis of local salvage therapies after radiotherapy for prostate cancer (MASTER). Eur Urol. 2021 Sep;80(3):280-292. doi: 10.1016/j.eururo.2020.11.010. Epub 2020 Dec 11. PMID: 33309278; PMCID: PMC10262981.
- Kissel M, Pounou A, Ka K, Alexis A, et al. Efficacy and toxicity following salvage high-dose-rate brachytherapy for locally recurrent prostate cancer after radiotherapy. Brachytherapy. 2022 Jul-Aug;21(4):424-434. doi: 10.1016/j.brachy.2022.01.005. Epub 2022 Mar 21. PMID: 35331666.
- Zamboglou N, Tselis N, Baltas D, et al. High-dose-rate interstitial brachytherapy as monotherapy for clinically localized prostate cancer: treatment evolution and mature results. Int J Radiat Oncol Biol Phys. 2013 Mar 1;85(3):672-8. doi: 10.1016/j.ijrobp.2012.07.004. Epub 2012 Aug 25. PMID: 22929859.
- Hoskin, PJ, Rojas, AM, Ostler, PJ, et al. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: Mature 12-year results, Radiotherapy and Oncology, Volume 154, 2021, Pages 214-219, ISSN 0167-8140, https://doi.org/10.1016/j.radonc.2020.09.047.
- Yamada Y, Rogers L, Demanes DJ, et al. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy. 2012 Jan-Feb;11(1):20-32. doi: 10.1016/j.brachy.2011.09.008. PMID: 22265435.
- Okamoto K, Okuyama K, Kohno N, et al. Clinical outcomes of low-dose-rate brachytherapy based radiotherapy for intermediate risk prostate cancer. J Contemp Brachytherapy. 2020 Feb;12(1):6-11. doi: 10.5114/jcb.2020.92405. Epub 2020 Feb 28. PMID: 32190064; PMCID: PMC7073334.
- Yan W, Chen J, Zhou Y, et al. Long-term outcome of early stage prostate cancer treated with brachytherapy analysis after a mean follow-up of 7 years. Springerplus. 2014 Jul 15;3:357. doi: 10.1186/2193-1801-3-357. PMID: 25089248; PMCID: PMC4117862.
How to Transform your practice with real-time HDR prostate brachytherapy?
Elekta eBook: Your Comprehensive Guide to Success
Are you a radiation oncologist looking to enhance your practice and broaden your treatment offerings?
Explore our latest eBook, Elekta’s Real-Time HDR Prostate Solution Solutions for Prostate Cancer, powered by Oncentra®, and revolutionize your approach to patient care.
Learn why this innovative treatment for prostate cancer is gaining interest among your peers and why it's chosen over traditional methods for its precision and effectiveness.
Whether you're already performing brachytherapy or new to the field, this comprehensive guide is your roadmap to success.
Content Overview:
- Why real-time HDR brachytherapy for prostate cancer?
- Conventional versus real-time HDR brachytherapy
- Recommendations on resources: equipment, personnel and facilities
- Getting started: educational programs with BrachyAcademy
- Go-live: Clinical consultancy & on-site Elekta support
Ready to lead the way in cancer treatment? Download our complimentary eBook and take the first step towards a brighter future for your patients.
Ready to implement Prostate Brachytherapy in your practice?
Book a meeting with our team to start HDR Prostate in your healthcare center.
Unlock exclusive content
Sign up and gain access to insights from radiation oncologists and physicists.
An asterisk (*) indicates a required entry.
