Optimized DCAT for lung and liver SBRT
Case: The introduction and widespread use of intensity modulated radiation therapy (IMRT) in recent decades has enabled improved radiation dose delivery in many cases, allowing better sparing of healthy organs and higher doses to the tumor.
Overview
But, for targets subject to motion (e.g. respiratory motion), the interplay between the movement of the MLC leaves and the target is a particular concern with IMRT techniques, especially when combined with the ablative doses used in stereotactic body radiation therapy (SBRT). The interplay effect may lead to under-dosage of the target volume or over-dosage of the surrounding healthy tissue. Recently, there has been renewed interest in the use of dynamic conformal arc therapy (DCAT) for certain SBRT treatments. DCAT reduces the issue of subvolume irradiation because the field aperture is dynamically changed to include the full projection of the target volume at each gantry angle. It has been proposed that the combination of DCAT with SBRT offers shorter treatment times and reduced MLC-target motion interplay effect. But, traditional DCAT does not offer the same level of plan quality that can be achieved using volumetric modulated arc therapy (VMAT).
Traditional DCAT uses a constant dose rate, which can lead to inferior dose distributions compared to VMAT. The dose is skewed higher where the distance to the target is shortest. This is particularly pronounced for targets that are off center axis.
To overcome this challenge, Monaco® treatment planning system (versions 5.1 and above) has a variable dose rate (VDR) feature for DCAT. VDR optimizes the DCAT delivery by allowing more or less dose to be delivered at any given gantry angle. Monaco changes the segment dose rate or the gantry speed to compensate for beam-to-target differences, which evens out the dose deposition and results in more conformal dose distributions.
Plus, the segment shape optimization (SSO) feature for DCAT in Monaco optimizes beam weights and shapes to enhance organs-at-risk (OAR) sparing and dose conformality. This function allows users to enter goals, constraints and optimization parameters so the planning system can calculate the best plan to meet these objectives. It does this by smoothing and clustering DCAT arc control points, and attributing more or less dose to each segment depending on the location and optimization priority of OAR in the beam path.
This case study presents 19 lung and liver SBRT patients.
Prescription
Nineteen patients, previously treated for lung (n=15) and liver (n=4) lesions with SBRT, were selected for this retrospective study. The Monaco treatment planning system (version 5.1) was used to optimize two SBRT plans for each patient, one using VMAT and one using optimized DCAT. The plans were created for Versa HD™ with a 6 MV FFF photon beam delivered in two 225-degree arcs.
Objective functions used during plan optimization for a sample case are shown in Table 1.
| Objective name | Objective function | Objective parameters |
| PTV | Target penalty | 99% of Rx dose |
| PTV + 4 cm | Quadratic overdose | 50% of Rx dose 1.2 cm from PTV with 5 cGy RMS |
| Ribs | Serial | 30 Gy |
| Body | Maximum dose | 130% of Rx dose |
Technique
The plans were optimized by a single dosimetrist using Monaco for Versa HD (6MV FFF) Two plans (one VMAT, one DCAT) were optimized for each patient using two 225° arcs and same PTV/OAR objectives.
To compare the the two plans, optimized DCAT versus VMAT, the following parameters were considered:
- The number of MU (correlated to treatment time)
- The conformity: ratio of PTV volume to 100% isodose volume (PTVr)
- The fall off: ratio of PTV volume to the 50% isodose volume (R50)
Regarding QA, a patient-specific QA using a Scandidos Delta4 phantom was performed, with the gamma index criteria being equal at 2% and 2 mm.
Device: Versa HD and Monaco 5.1
Metrics
Figure shows comparison between optimized DCAT (top) and VMAT (bottom) isodose distributions demonstrating comparable plan quality (Fig. 1).
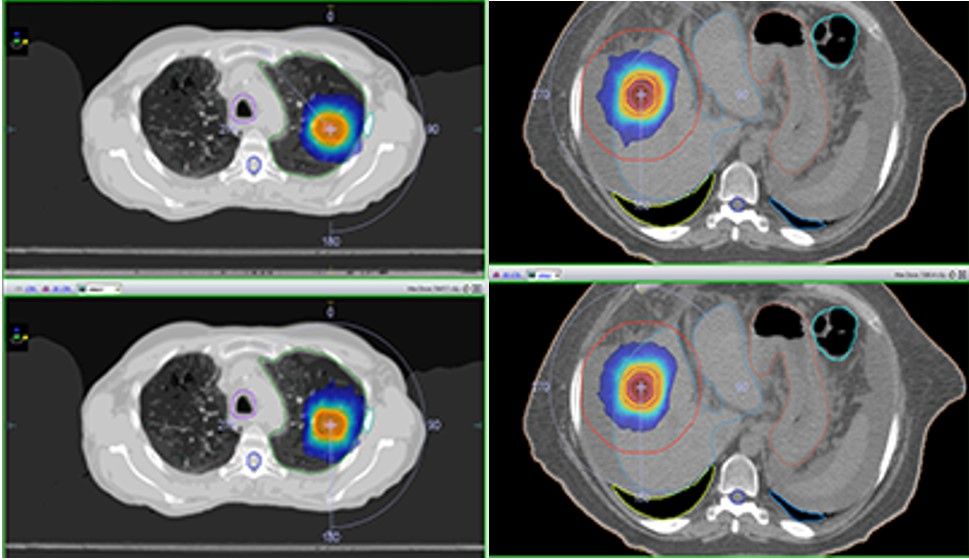
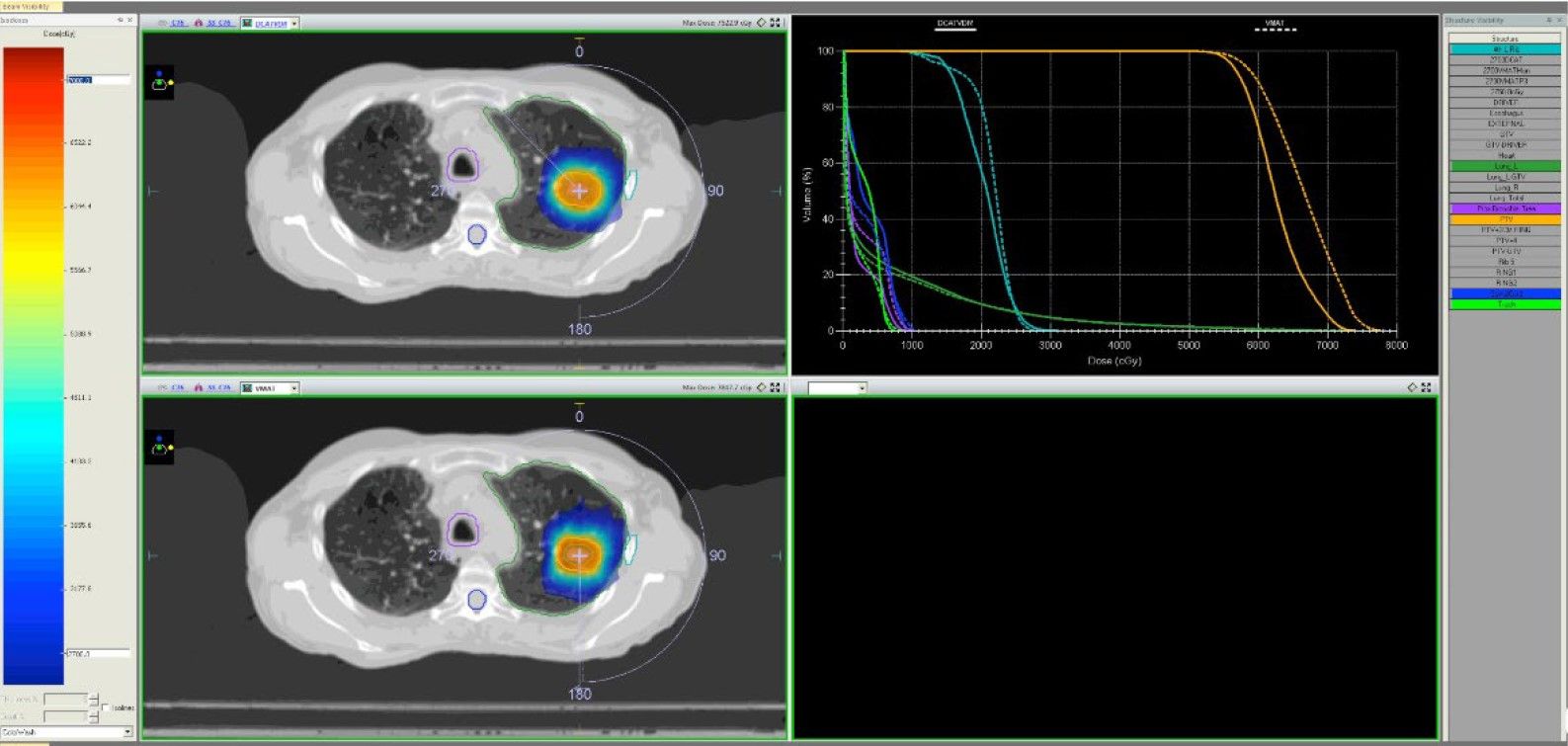
Technical Planning Comments
All VMAT and optimized DCAT plans achieved the planning objectives. Although target coverage was slightly better for the VMAT plans (VMAT average PTVr = 1.3; DCAT average PTVr = 1.4), this was not shown to be statistically significant. Similarly, average R50 values for VMAT and DCAT were very close (4.5 and 4.6, respectively).
Overall, optimized DCAT plans showed inferior plan quality compared to the VMAT plans when OAR overlapped or were in close proximity to the PTV volume, but showed comparable or superior quality when targets were far from OAR.
The average number of MU in optimized DCAT plans was reduced by a factor of 2.5 compared to VMAT plans (Table 2).
| VMAT | Optimized DCAT | VMAT:DCAT ratio | |
| Average MU | 5,548.7 | 2,277.9 | 2.52 |
| Standard deviation | 2,077.9 | 991.1 | 1.0 |
| Range | 1,647-8,062 | 948-3,955 |
All VMAT and DCAT plans were accurately delivered on a static IMRT QA phantom and had excellent gamma index passing rates, with >95 percent of evaluated points within the gamma index criteria.
With DCAT, the beam aperture is almost always open to cover the entire PTV, so the MU per fraction is reduced compared to VMAT and static IMRT deliveries. Since the number of MU correlates to delivery time, we have shown that optimized DCAT lung and liver SBRT plans could be delivered on average 2.5x faster than VMAT plans, with generally similar plan quality. The ability to vary dose rate and gantry speed, combined with the ability to spare OAR with SSO, enables optimized DCAT to provide highly conformal dose coverage comparable to VMAT plans. Plus, since DCAT plans produce a more homogenous delivery fluence by nature, and the MLC leaves do not block the PTV during delivery, the interplay effect between MLC motion and PTV motion is minimized.
Based on the results of this study, lung and liver SBRT patients with simple, spherical lesions that are not close to OAR are ideal candidates for optimized DCAT. At our institution, this would mean that 40–60 percent of SBRT patients who would normally be treated using VMAT could be treated more efficiently using optimized DCAT, while maintaining plan quality.
These patients could benefit from significantly shorter treatment times, which are easier to tolerate and reduce the risk of intrafraction movement. VMAT would still be the preferred option for cases where OAR (such as ribs or heart in lung cases, or stomach in liver cases) are very close to or overlap the PTV.
The average DCAT MU in this study was around 2,300, which would take less than two minutes to deliver both arcs using a 6 MV FFF beam (1,200 MU/ min). The shorter treatment times achieved with optimized DCAT are favorable for the deep inspiration breath hold (DIBH) technique. DIBH would further benefit patients by allowing reduced ITV and PTV volumes and improved healthy tissue sparing.
In conclusion, optimized DCAT offers faster treatments for lung and liver SBRT patients, while generally maintaining plan quality.
Report
“Lung and liver SBRT patients with simple, spherical lesions
that are not close to OARs are ideal candidates for optimized
DCAT.”
Dr. Sotiri Stathakis, University of Texas Health San Antonio, USA