Complex, two-target SABR lung treatment using Monaco® and Symmetry™
Summary
Monaco® treatment planning and Symmetry 4D IGRT allow highly conformal, accurate, efficient and safe SABR treatment of tumors in both lungs.
| Contributors: | |
Mr. Peter Enever Mrs. Laura Jackson Mrs. Eleanor Quinn | |
| Patient demographics: | Treatment: |
|
|
| Diagnosis: | Treatment planning and delivery system: |
|
|
Diagnosis
Patient history and diagnosis
A 62-year-old female patient was diagnosed with idiopathic dilated cardiomyopathy with severe left ventricular systolic dysfunction toward the end of 2018. During investigations, nodules were identified in both lungs. CT-guided biopsy of the right lower lobe nodule revealed a squamous cell carcinoma stage T1a N1 M0 (8 mm diameter).
The left lower lobe nodule received a radiological diagnosis of cancer stage T1b N0 M0 (15 mm diameter). The patient was unsuitable for surgery due to her idiopathic dilated cardiomyopathy and the need to remove the lower lobe of the right lung if surgery were utilized. Since patient performance status was 0, lung SABR was deemed a reasonable option. The alternative would have been a standard dose and fractionation (e.g., 50 Gy in 25 fractions) VMAT treatment with larger margins and a potentially lower overall dose.
Challenges
Treating nodules in both lungs requires a more complex treatment plan due to the risk of increased organ-at-risk (OAR) dose. Onset imaging shifts also need to be considered prior to treatment delivery so dose to the contralateral PTV and OAR is not inadvertently increased. A compromise between PTV coverage and OAR dose may need to be considered during planning, imaging and treatment—so the treatment solutions used must have this capability.
When tumors are at different levels in the lung, two separate 4D simulation scans may be required and the additional dose considered. Even when both tumors can be incorporated in a single scan, imaging data can be corrupted by abnormal breathing. In this case, since both tumors were in the lower lobes, they were covered in one scan and the breathing trace was good.
The tumors were in close proximity to each other, as well as the spine and major blood vessels, which had to be accounted for during planning. A combined plan was used to accurately assess dose to the OAR and each target, as described below.
Treatment planning
RTOG protocol 0236 guidelines were used in planning this patient’s treatment.[1] A total of 50 Gy delivered over eight fractions (6.25 Gy per fraction) was prescribed for each tumor and was defined by OAR dose, mainly to the spinal canal. Leeds lung SABR protocols have four dose prescriptions based on the RTOG protocol 0236, of which this is the lowest.
The patient’s thorax was immobilized with the patient positioned lying supine with arms above her head, utilizing a Bionix wingboard immobilization device. A Klarity Medical vacuum bag was added under the arms within the wingboard to aid arm comfort and stability, reducing the risk of patient movement. A thin mattress was placed under the patient’s back and a knee block was used to increase comfort and leg stability.
For simulation, a 4D CT scan plus contrast was obtained (Phillips CT Big Bore with Phillips Brilliance CT software powered by iPatient), with the patient breathing normally and respiratory motion tracked throughout using a “diaphragm belt.” Intravenous contrast was delivered during an initial 3D scan and a 4D scan was performed immediately after. Dose constraints reduced scan length for 4D, therefore a 3D scan was obtained to ensure the lungs were fully imaged for dose mapping. When 4D scan quality is poor, it is either repeated or the 3D scan used for planning.
The ability to capture a 4D planning scan allows the MDT to utilize this in ITV delineation, potentially reducing the total margins. This information, when combined with Symmetry, enables real-time assessment of tumor motion.
Monaco version 5.11 was used to plan this lung SABR treatment. A combined plan (Figures 1-2) was created with two 6 MV FFF VMAT arcs so the total dose to all OAR and both tumors could be assessed accurately. The spinal canal, located between the two tumors, would receive dose from each tumor treatment. A combined plan, planned simultaneously with two treatment arcs, gave a more exact indication of total dose to the spine, which was selected to receive a maximum dose. The flexibility of the Monaco system allowed this. Due to their distance from the tumors, the heart, bronchial tree and esophagus did not receive significant dose, and use of IGRT allowed us to closely monitor proximity to OAR. The V20 dose constraint exceeded the optimal value of 6% due to the two tumor sites being treated, but was still well within mandatory levels and therefore caused no dose issues.
Two separate isocenters were used in this instance because the tumors were located at different levels in both lungs. This option also reduced the potential of having to use an offset isocenter, which can result in excessive segments being created and can reduce the ability to perform a full 360 XVI scan due to clearance.
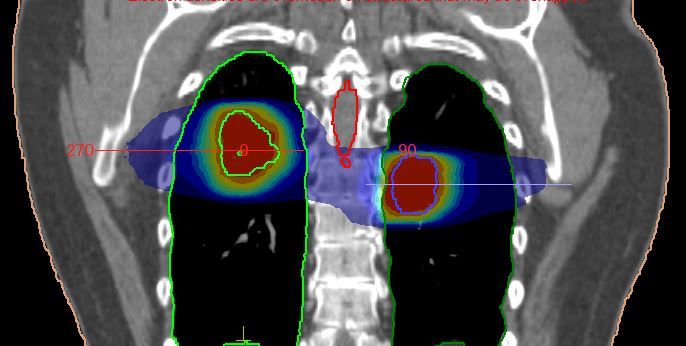
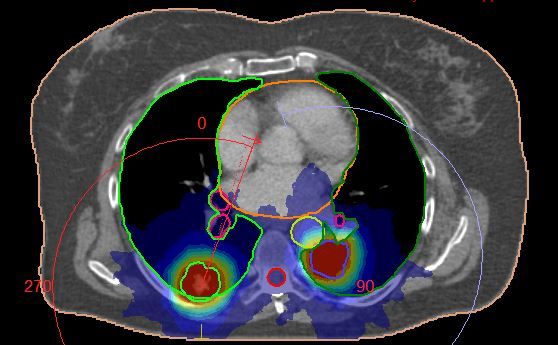
The PTV for each tumor was defined by applying a 5 mm margin to the ITV in all directions. In this case, the lowest SABR protocol total treatment dose had to be used due to the limiting dose constraint of the spinal canal.
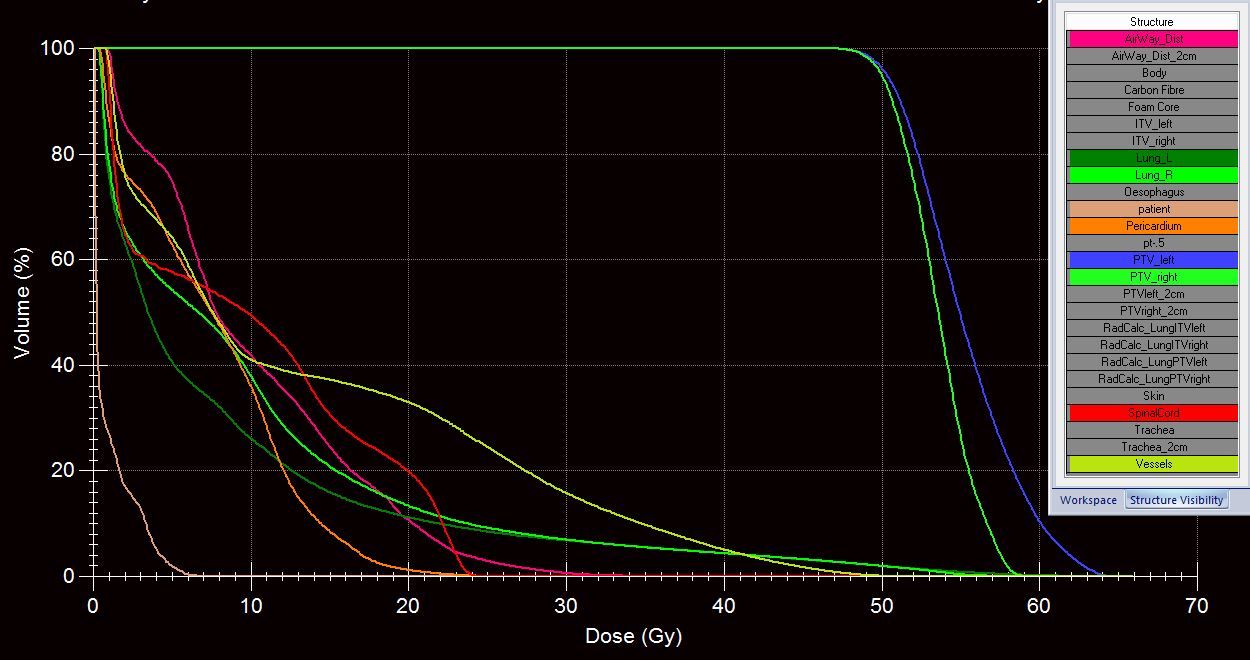
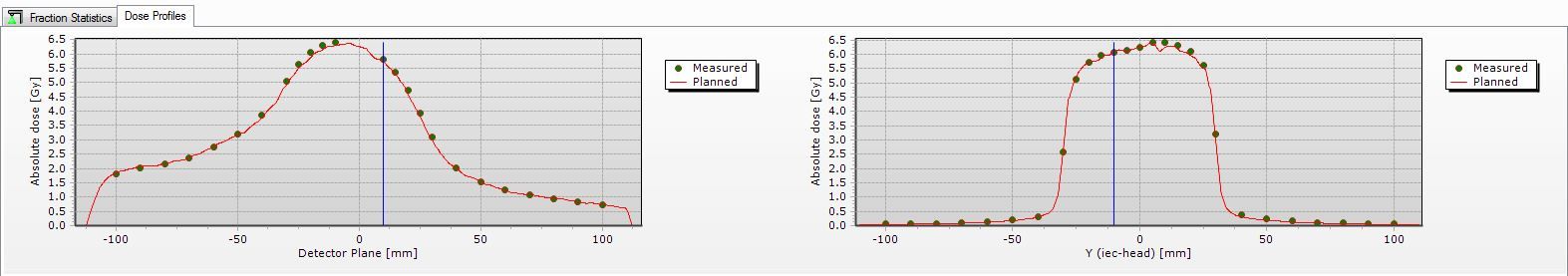
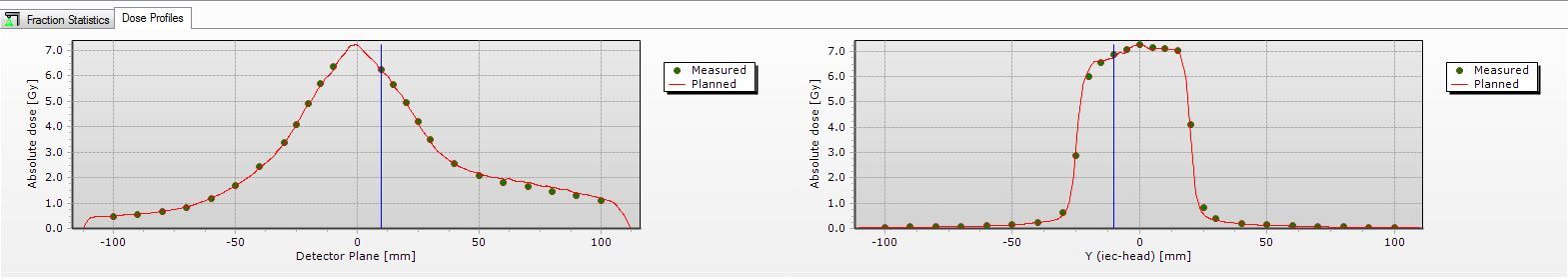
Quality assurance (QA) was performed using a Delta4 two-diode array and a CIRS lung phantom ion chamber. Each treatment arc had individual QA performed prior to first treatment delivery. Delta4 automatically compared the delivered dose with the planned dose (Figure 3). In addition, monitor units, MLC leaf position, gantry and collimator angle were measured and independently verified.
Treatment delivery
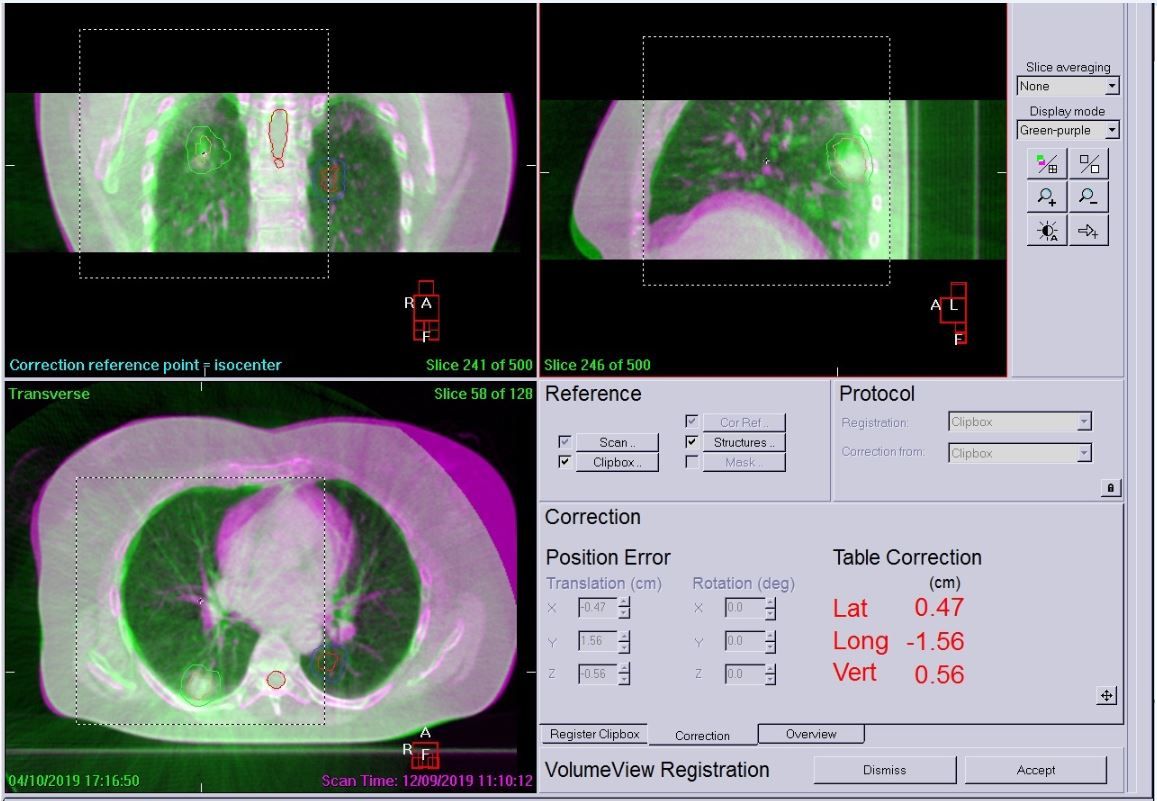
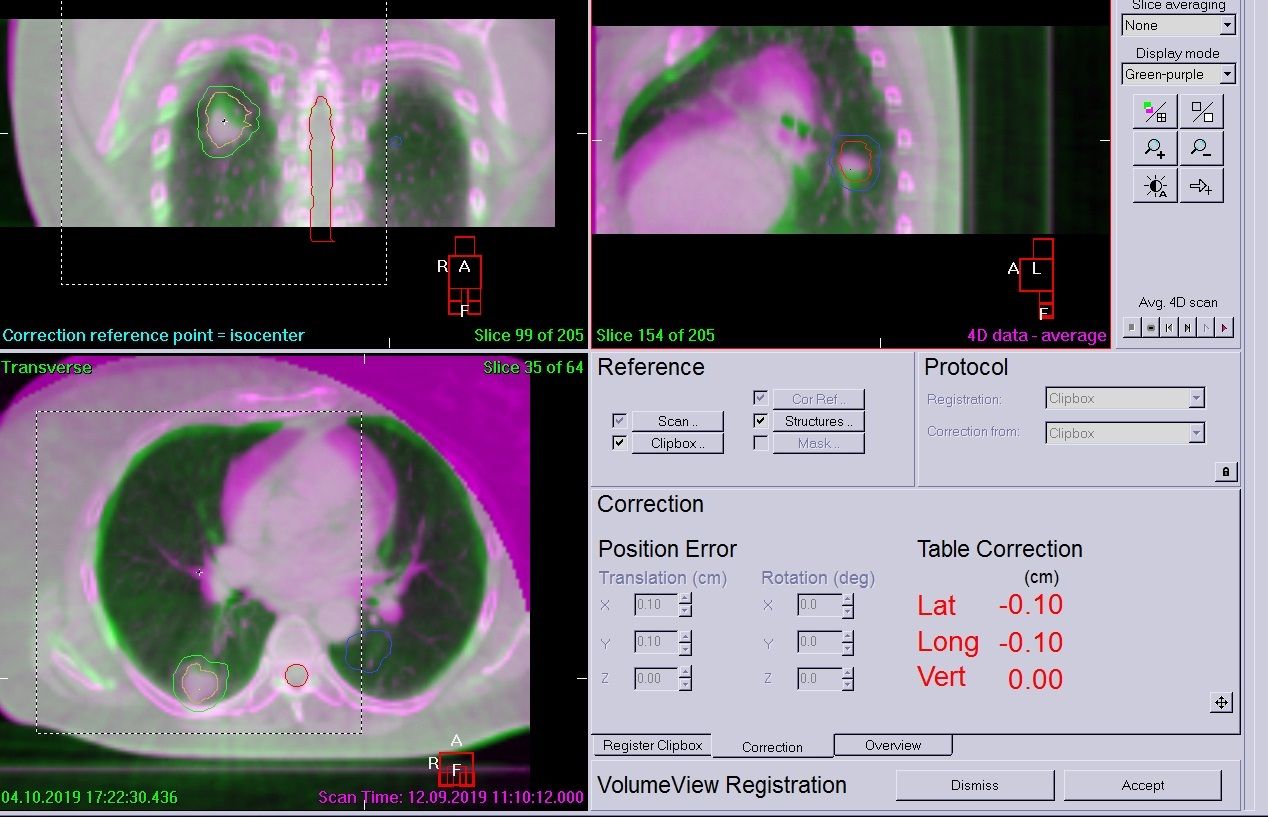
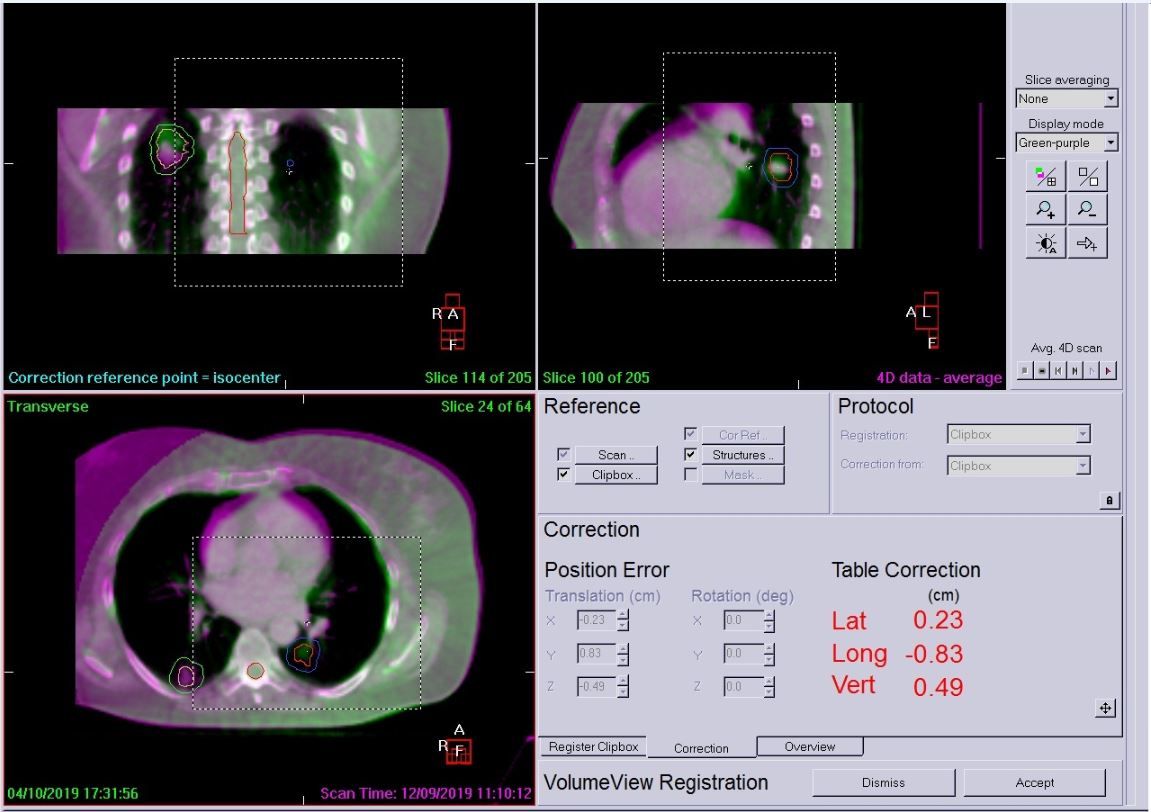
The patient was positioned as for simulation and was free-breathing for all treatments. A Symmetry 4D CBCT scan was used on day zero to assess breathing, tumor motion and whether a 4D scan was needed each day (3D can be used if there is very minimal tumor motion). In this case, the patient required all scans to be 4D for both tumors due to breathing motion and the complexity of the plan. This would not have been possible without the ability to use Symmetry 4D image guidance. A 4D XVI scan was performed prior to treatment of each tumor to account for breathing and tumor motion, accurate coverage of the GTV and to ensure that any shifts required did not increase dose to OAR or the contralateral tumor.
Both tumors were treated on the same day with matching to the spine (as the closest OAR receiving dose from both treatment arcs). The right tumor was treated first each day so daily matching was consistent.
Following daily imaging, if errors were over 2 mm, a correction was applied and then another scan performed to check for further movement. The patient was very compliant and only two 4D scans were required before each treatment. Example LFOV and MFOV scans are shown in Figures 4–6. All imaging schedules are based on Leeds protocols that use SABR consortium guidelines as a reference,[2] and retrospective audit of SABR treatments.
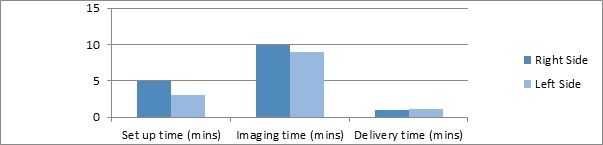
Each treatment area involved one arc, taking approximately one minute to deliver. Including imaging to ensure accurate treatment delivery, the average time on the table was less than 40 minutes to treat both areas (Figure 9). The immobilization devices used were crucial in aiding patient comfort and compliance. The patient coped very well and no avoidable patient movement was observed.
Outcome & Follow up
Six weeks after treatment, the patient had recovered from early toxicities, which consisted of esophagitis and some neuropathic pain. There was no weight loss and she had returned to eating normally. Her most recent x-ray showed no concerns of radiation pneumonitis. A restaging CT was scheduled for 6 months post-treatment with a clinic review.
Prior to the 6-month follow-up date, the patient was referred for spine MRI due to pelvic discomfort. MRI and restaging CT showed metastases present. Comparison measurements showed that the lung nodules in the right and left lower lobes had decreased in a size by 5 mm and 9 mm, respectively. The patient was referred for further palliative radiotherapy.
Discussion
In a non-randomized comparison of two prospective cohorts of medically inoperable stage I lung cancer patients, overall survival and local control were better after SABR compared to 3D-CRT. Global quality of life, physical functioning and patient-rated dyspnea were stable after SABR, whereas physical functioning decreased after 3D-CRT, approaching clinical significance already at one year.[3] SABR was chosen in this instance because the patient was deemed high risk for surgery due to her idiopathic dilated cardiomyopathy. MDT discussion determined that both tumors should be treated on the same day in order to closely monitor spinal cord position. Initially, queries were raised whether the left-sided mass could be treated with SABR due to the proximity of OAR (i.e., the airways). However, once planned, it was decided that 50 Gy delivered over 8 fractions using a combined plan was the best course of action.
The Monaco treatment planning system is able to provide high quality radiotherapy treatment plans for a wide range of radiotherapy techniques, including SABR.[4] Monte Carlo dose calculations in Monaco provide gold standard accuracy, particularly for inhomogeneous tissue densities. This planning system also provides the ability to plan with isoconstraints, distinguishing parallel and serial organs (in this case lung and bronchial tissue) and applying biological optimization.
When leveraging Monaco to utilize the rapid leaf speeds of the MLC’s, higher dose rates can be used for more effective modulation. This offers greater OAR sparing,[5] while the MLC’s extremely low transmission (< 0.5%) and the Y jaw’s dynamic ability to make 1 mm increments reduces unwanted dose to healthy anatomy. The use of a flattening-filter free (FFF) beam also reduces scatter, further sparing healthy tissue and reducing dose to nearby OAR.
With high-speed MLC leaves supporting higher deliverable dose rates, FFF enables faster delivery speeds. Treatment times were shown to have reduced by 61% for 10 MV FFF and 55% for 6 MV FFF beams when compared to 6 MV flattened beams.[6] A comparable treatment using 6 MV flattened beams would have taken around 40 minutes for each tumor site, compared to the 40-minute or less total treatment time for both areas in this case. The speed at which Versa HD is able to deliver treatments enhances patient compliance and reduces the risk of unnecessary intrafraction movement.
Symmetry 4D CBCT scanning at the time of treatment increases confidence in the accuracy of SABR treatment delivery. Although margin reduction was not utilized for this plan due to tumor motion, the margins used were still smaller than those used for standard dose VMAT lung radiotherapy (0.5 cm margin versus 1 cm margin). The ability to acquire two scans within minutes of each other, make the necessary couch position corrections from outside the treatment room and then immediately proceed to treatment is vital when delivering such a conformal and high dose treatment, especially when nearby OAR are reaching their maximum dose point. Both PTVs could be viewed using Symmetry, which allowed us to monitor spinal cord dose closely, and the 4D imaging capability ensured that coverage was maintained during the full range of breathing motion.
Conclusion
The Monaco treatment planning system enabled us to successfully plan a complex, two-target SABR lung treatment and to accurately assess OAR dose. The Symmetry 4D IGRT capability of Elekta linear accelerators allowed accurate visualization of tumors and OAR, and the external contour of the patient using the LFOV collimator. Elekta’s Versa HD supported fast delivery of a high-dose conformal treatment with great accuracy and reproducibility. Delivery of such a highly conformal, accurate, efficient and safe treatment is beneficial for tumor control and an increased quality of life for lung cancer patients.
References
- Xiao Y, Papiez L, Paulus R, et al. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: Stereotactic body radiotherapy of inoperable stage I-II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73(4):1235-1242.
- SABR consortium Guidelines 2019 version 6.1.
- Widder J, Postmus D, Ubbels JF, et al. Survival and quality of life after stereotactic or 3D-conformal radiotherapy for inoperable early-stage lung cancer. Int J Radiat Oncol Biol Phys. 2011;8(4):e291-7.
- Clements M, Schupp N, Tattersall M, et al. Monaco treatment planning system tools and optimization processes. Med Dosim. 2018;43(2):106-17.
- Sahgal A, Ruschin M, Lee Y. A comparison of VMAT plan quality (target coverage and healthy tissue sparing) and the impact of MLC design for Elekta Agility and Varian HD120. Sunnybrook Health Sciences Centre. Elekta website case study.
- . Tambe NS, Fryer A, Marsden JE, et al. Determination of clinically appropriate flattening filter free (FFF) energy for treating lung SABR using treatment plans and delivery measurements. Biomedical Physics & Engineering Express. 2016;2(6):065016.