RTT-contouring streamlines MR-Linac workflow at UK’s The Royal Marsden

Reallocation of clinician’s task boosts overall workflow efficiency, enriches RTT skills, lowers costs and increases Elekta Unity MR-Linac capacity

While the introduction of high-field MR-guided radiation therapy (MRgRT) has revolutionized the field of radiation therapy, it came at the price of longer treatment times and more intensive involvement of the radiation oncologist versus conventional linac radiotherapy. However, at many Elekta Unity MR-Linac sites, the solution was to recruit key members of the radiotherapy team – radiographers/radiation therapy technologists (RTTs) – to relieve radiation oncologists from target and organs-at-risk (OAR) contouring of selected cases. Since 2021, the UK’s The Royal Marsden NHS Foundation Trust (RMH) has demonstrated that RTT contouring for MR-Linac cases – prostate and bladder so far – is a winning formula for streamlining workflow and reducing costs.
Radiographer contouring for Unity cases is actually an expansion of what some RMH RTTs have been doing for several years for prostate cases on the center’s seven traditional linacs. Urology specialist RTTs have been upskilled to review cases, see and consent patients in the clinic, and draw all contours on CT images for around 50 percent of all prostate patients, according to RMH Radiation Oncologist Angela Pathmanathan, MD.
“From our point-of-view it wasn’t strange for us to think that contouring on Unity should be done by RTTs as well.”
“We ultimately review their contours for the final sign off, but these RTTs are doing a significant amount of the initial contouring,” she says. “Medical residents will do some contouring, but they can’t do them all due to their clinic obligations. From our point-of-view it wasn’t strange for us to think that contouring on Unity should be done by RTTs as well. We knew that once they had a certain degree of training they would be comfortable using MR images as well – it was a natural progression from our side.”
Upskilling skilled RTTs
In 2020, clinicians led by Dr. Pathmanathan began the process of upskilling RTTs to contour Unity cases, starting with instruction on MRI principles and safety, basic anatomy and the essentials of contouring volumes. This was followed by having RTTs contour cases offline with clinician feedback. Subsequently, RTTs contoured in an online supervised scenario, in addition to more offline contouring practice – all before RTTs were cleared to contour unsupervised.
“It was and is quite an extensive training program,” she says. “We didn’t want the RTTs to feel that they’re being thrown into the deep end of the pool.”
RMH Senior 1 MR-Linac Therapeutic Radiographer Bethany Williams, RTT, observes that multidisciplinary collaboration in RTT contouring training is crucial.
“Quality training materials were a vital part to ensure we could achieve accurate and consistent target volume contouring between RTT observers,” she explains. “The clinical oncologists played a huge part in creating a dynamic library of PowerPoints, videos and guides for our RTTs. They review and sign off on the offline and online RTT contours on a one-to-one basis. We now also include competent and experienced radiographers in the training program who supervise other RTTs under training as well as guiding new doctors.”
The result of the RTT training has been to remove the clinical oncologist from the online contouring workflow, as well as adaptive treatment plan approval.

“To accomplish that, our medical physicist team and clinical oncologists created a set of criteria – online dosimetric constraints – for what could be accepted online,” Williams observes. “It specifies dose constraint violations that can be accepted or that enable a delegated operator such as a physicist to approve a treatment plan. Or, in the case of our ‘RTT-led’ workflow for partial breast irradiation, it will allow an RTT to approve a treatment plan.”
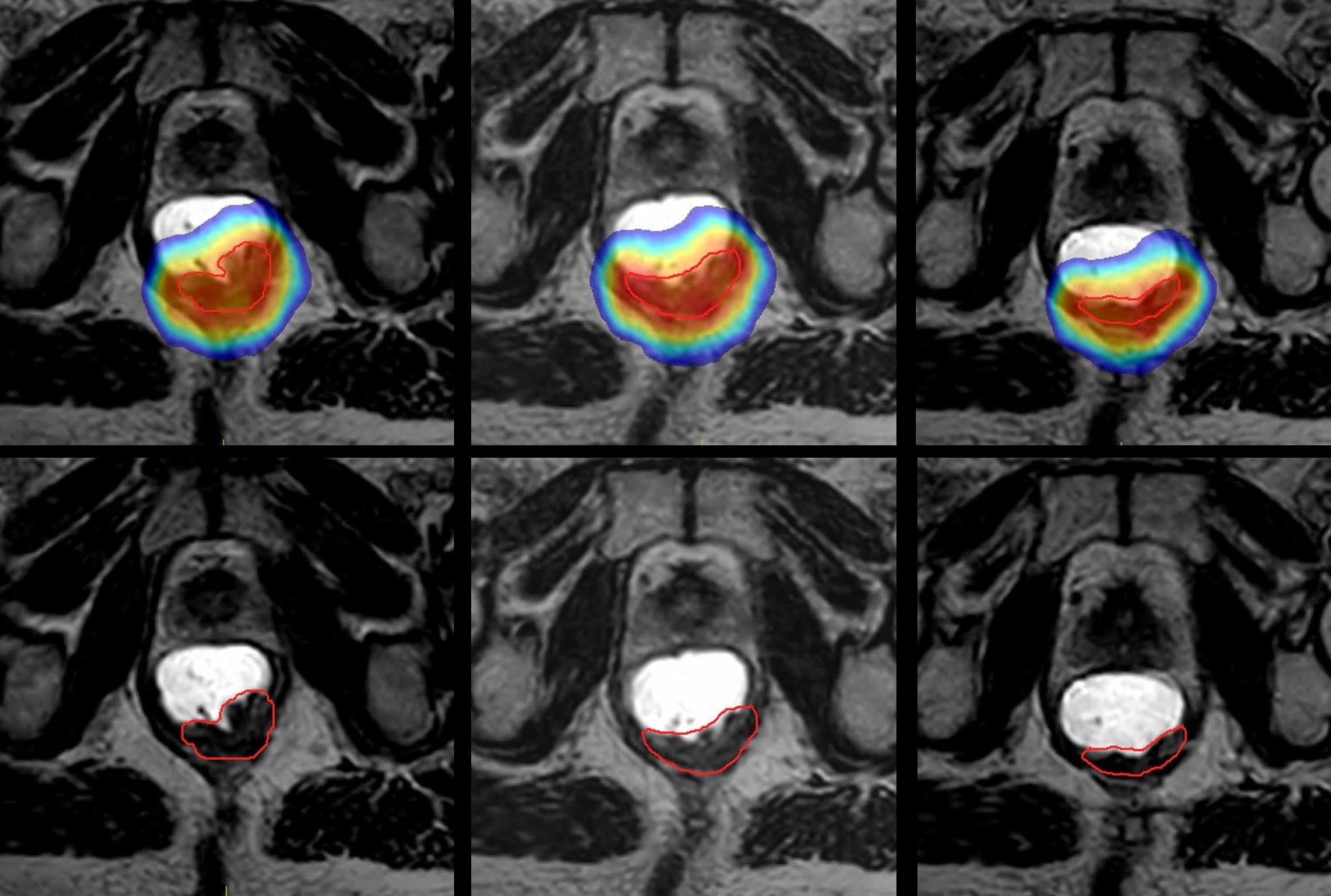
To validate the robustness of the RTT contouring training for Unity cases, RMH RTTs and radiation oncologists engaged in a study that compared the contouring performance of RTTs versus clinical oncologists in MRgRT-guided online adaptive prostate radiotherapy.1 Volume metrics included the Dice similarity coefficient (DSC), mean distance to agreement (MDA), Hausdorff distance (HD) and dosimetric analysis to evaluate contours.
“Among the most common questions we’re asked regarding RTT contouring is whether clinicians review our contours offline. They don’t, because we’ve implemented this robust training program that is validated by these evaluations and clinical audits.”
“The result was excellent agreement between clinician contours and RTT contours,” notes Williams, a study co-author. “Among the most common questions we’re asked regarding RTT contouring is whether clinicians review our contours offline. They don’t, because we’ve implemented this robust training program that is validated by these evaluations and clinical audits.”
RTT contouring in practice at RMH
Today, RTTs contour the majority of RMH's prostate and whole bladder workflows using daily re-contouring and online plan adaptation to fully leverage the capabilities of the online adaptive workflow. They also lead the workflow for partial breast in which they perform plan re-optimization and plan checks.
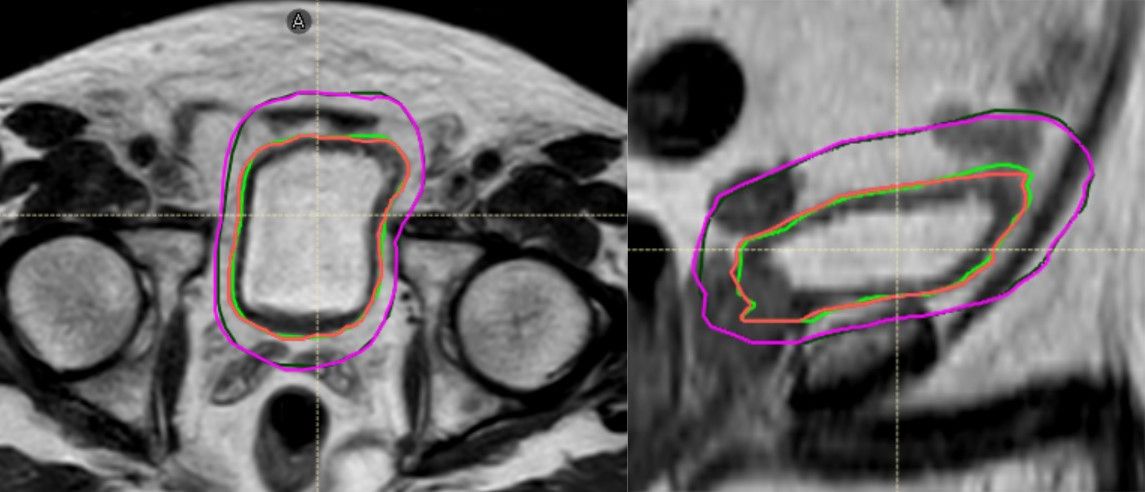
“It’s worth noting that all of our 20-fraction prostates and five-fraction SBRT prostates are contoured by RTTs unless they’re deemed very complex,” Williams says. “Regardless of the treatment site, a clinical oncologist is currently required to contour the first treatment fraction, which we use as a reference plan for all subsequent fractions.
“Having said that, we are now exploring moving away from that practice, providing that the reference plan is an MR image,” she adds. “Doing this would help further reduce resource intensity.”
Treatment times for 20-fraction prostate radiotherapy range from 35 to 45 minutes and 45 to 60 minutes for the five-fraction prostate SBRT, both of which benefit from Elekta’s Comprehensive Motion Management (CMM) automatic gating.
“We went live with CMM in June 2024,” Williams says. “The ability to automatically gate the patient’s treatment ensures accuracy of treatment delivery and the opportunity to apply baseline shifts when the target moves outside of the PTV [e.g., due to rectal distention or rectal gas], significantly reducing the number of partial treatments. During online workflow for all sites, CMM is RTT-led from template acquisition and approval to applying and approving baseline shift plans.”
Significant cost- and time-savings realized with a new way of working
Shifting the contouring responsibilities to the RTTs for online prostate and bladder cases has reduced the number of staff needed at the treatment console.
“By not requiring the presence of a clinical oncologist for bladder and prostate adaptive radiotherapy – the latter comprising about 50 percent of Unity utilization in a year – means we save a lot of time by not having to organize these treatments around the clinical oncologists’ limited availability due to their clinics, meetings or schedules,” Williams says. “That gives us greater flexibility when booking appointments, which not only enhances the patient’s experience, but also increases Unity capacity for other treatment sites that do require a doctor’s presence.”
At ESTRO 2024, Williams presented on behalf of London City University research assistant Emma Xue, who collaborated with Williams to conduct a cost-consequence analysis**† for the year 2022-2023. The analysis explored clinician time reductions and cost-savings in both 20- and five-fraction prostate treatments compared with clinician contouring, in addition to the impact on patient throughput. The results:
- The time-savings for “clinician attendance” were approximately 700 hours (20 fractions) and 180 hours (five fractions). For “clinician active” time, around 500 hours (20 fractions) and 100 hours (five fractions).
- The analysis estimated that RTT-contoured workflows for prostate MRgRT lead to a cumulative cohort saving of approximately £80,000-110,000 (USD $101,900-140,100) and £19,000-25,000 (USD $24,200-31,800) for the 20-fraction and five-fraction treatment courses, respectively. This equates to a per-patient cost reduction of about £1,000-2,000 (USD $1,274-2,547) for 20-fraction and around £300-400 (USD $380-510) for five-fraction treatments.
- Post-PACE-B2, referrals for a 36.25 Gy X 5 course are expected to increase. The total annual cost-savings for hypofractionated treatment courses is anticipated to be greater due to additional capacity to treat more patients. In analysis of cohort size, using clinical service hours to estimate the MR-Linac’s capacity – and accounting that prostate treatments represent 53.2 percent of its use – there is an increase in annual patient throughput based on the greater availability of RTTs versus clinical oncologists. Specifically, RTT-contoured workflows boosted capacity for about 130 patients treated with the five-fraction course compared to clinical oncologists’ availability. In this scenario, the model estimated that RTT contouring would result in a total cohort saving of over £70,000-90,000 (USD $89,100-114,600) yearly for patients prescribed five fractions. The model also estimated this would save clinical oncologists 700 hours.
- Clinical increase in capacity: RMH used to allow for a maximum of three patients to receive prostate radiotherapy on the MR-Linac in one clinical day as a result of the limited availability of clinical oncologists. RMH now has the capacity for up to eight (potentially 10) patients depending on the complexity of cases and referrals for other treatment sites.
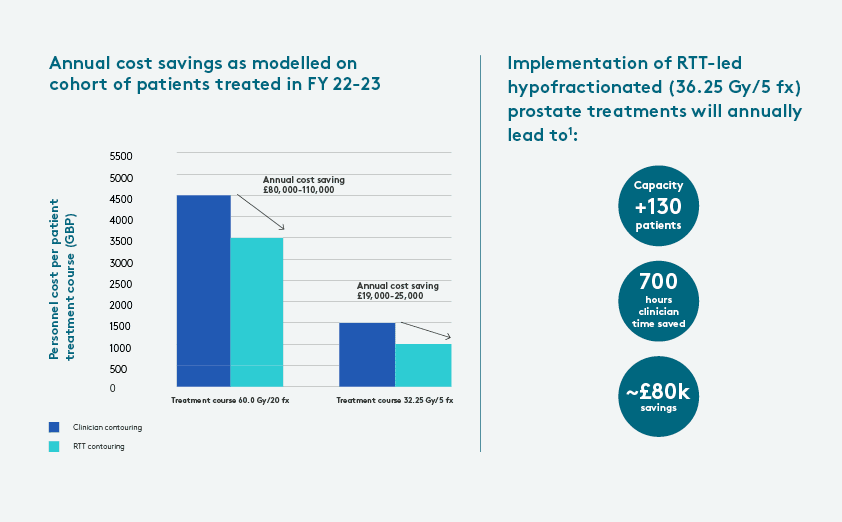
1 As compared to workflow with clinician contouring and combination of 20 fx and 5 fx treatments and as modeled on maximum MRL utilization, where 50% of patients treated receive prostate radiotherapy (based on average prostate activity).
RTT career enrichment could enhance recruitment and retention
With RTT contouring for prostate and bladder indications well-established, RMH has begun training RTTs to contour pelvic oligometastases cases, with RTT training for rectal, kidney, liver and pancreas cases on the horizon. Williams stresses that the center wants to ensure that it has at least three to four RTTs trained competently in a given site before implementation and anticipates that members of the MR-Linac Consortium can offer support.
“Consortium centers, such as University Medical Center Utrecht, Odense University Hospital and Princess Margaret Hospital have demonstrated excellent online workflows for which RTTs do contouring and ATS re-optimization,” she notes. “And the Christie Hospital NHS Foundation Trust has done some work on OAR RTT contouring for pancreas. Through collaboration within the Consortium, we will be able to develop the RTT’s role.”
In the face of aging populations and an ever-increasing demand for radiotherapy, however, the key to advancing the RTT’s role in MRgRT is a continued influx and retention of RTTs in radiotherapy departments, Williams points out.
“With a global shortage of RTTs, investment in training and retention of the RTT workforce is vital, particularly in this adaptive space in which the skills are so specific.”
“With a global shortage of RTTs, investment in training and retention of the RTT workforce is vital, particularly in this adaptive space in which the skills are so specific,” she says. “My colleagues, Elizabeth Joyce and Helen McNair, did a training needs analysis3 and they found that the highest priority need for training in adaptive radiotherapy is MRI acquisition, MRI contouring and treatment planning.
“I’ve observed from conference presentations on the topic and the wave of emerging literature,” Williams continues, “that expanding the scope of RTTs will contribute to making the career more appealing and, most importantly, support RTT retention in the current workforce.”
For her part, Williams needs no convincing that the growing role of RTT in MRgRT makes it worthwhile to stay in the field.
“I thoroughly enjoy working in the forefront of adaptive radiotherapy,” she says. “It’s incredibly engaging to work with MRI, a very complex imaging modality, and the responsibilities related to adaptive radiotherapy – the contouring and adaptive planning. And the field is ever advancing. I feel incredibly lucky to be part of the Consortium and it’s these RTT workflows – the simulation-free workflows and the integrated functional imaging – it’s definitely a fascinating area that will become more sophisticated and more beneficial to patients in their treatment outcomes.”
Dr. Pathmanathan agrees and is “highly positive” about the RTTs’ continually evolving role for both conventional linac and MRgRT contouring.
“We as radiation oncologists will still be involved in the process, but not spend nearly as much time when we have clinical obligations and other things to do,” she says. “It has been very much about the natural progression of the role of our radiographers, who have proven to be highly motivated to contribute their skills and abilities and who have made this initiative very successful.”
Click here to learn more about MRgRT with Elekta Unity..
** Based on the UK’s Health Economics Standards for the National Health Service (NHS).
† This independent research was funded by the National Institute for Health Research and Health Education England (HEE/ NIHR ICA Programme Senior Clinical Lectureship, Dr Helen McNair, ICA-SCL-2018-04-ST2-002) and supported by the NIHR Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London. The views expressed are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social.
References
- Adair Smith G, Dunlop A, Alexander SE, et al. Evaluation of therapeutic radiographer contouring for magnetic resonance image guided online adaptive prostate radiotherapy. Radiother Oncol. 2023 Mar;180:109457. doi: 10.1016/j.radonc.2022.109457. Epub 2023 Jan 3. PMID: 36608770; PMCID: PMC10074473.
- van As N, Griffin C, Tree A, et al. Phase 3 Trial of Stereotactic Body Radiotherapy in Localized Prostate Cancer. N Engl J Med. 2024 Oct 17;391(15):1413-1425. doi: 10.1056/NEJMoa2403365. PMID: 39413377; PMCID: PMC7616714.
- Joyce E, Jackson M, Skok J, et al. What do we want? Training! When do we want it? Now? A training needs analysis for adaptive radiotherapy for therapeutic radiographers. Radiography (Lond). 2023 Jul;29(4):818-826. doi: 10.1016/j.radi.2023.05.015. Epub 2023 Jun 16. PMID: 37331130.
LARMRL250129