Novel AI-enhanced imaging will expand access to adaptive radiation therapy
How Elekta is paving the way for high definition, AI-enhanced CBCT imaging
In 2003, Elekta introduced the world’s first image-guided radiation therapy (IGRT) system, which integrated a digital linear accelerator with a high-resolution cone beam CT (CBCT) imaging workflow. This innovation ushered in an era in which the clinical team could acquire 3D images with the patient in the treatment position – significantly reducing uncertainty regarding the position of OARs and cancer targets. Although Elekta linacs would benefit by numerous technological enhancements (e.g., beam-shaping, 4D imaging, flattening filter-free delivery) in the ensuing years, CBCT image quality remained largely unchanged. With recent developments, however, CBCT image quality – as well as quantitative imaging results – are poised to break new ground again.

“The standard of care in radiotherapy has been to acquire a cone beam CT image right before treatment and use that image of the day to account for any rigid shifts of the patient,” says Elekta Senior Scientist Jonathan Mason, MEng, PhD, a member of Elekta’s Clinical Application Development team, a group of scientists, clinical experts and experienced engineers who work together to define future imaging, planning and treatment delivery technology. “But what if you could see the targets and organs-at-risk even better with greater image quality? That would allow you to personalize and adapt the therapy even more and reduce toxicity – delivering a better patient treatment from fraction to fraction.”
To that end, Dr. Mason and his colleagues have worked to develop an AI-driven scatter correction algorithm and a next generation iterative reconstruction algorithm, the latter which evolved from Dr. Mason’s 2018 doctoral thesis. Together, these innovations are designed to significantly improve CBCT image quality and dose planning accuracy, in turn facilitating adaptive, personalized radiotherapy for a greater number of cancer patients.
“It’s very satisfying to see how my thesis has evolved into a key part of a clinical innovation that has the potential to benefit so many clinics,” Dr. Mason.
High definition, AI-enhanced imaging
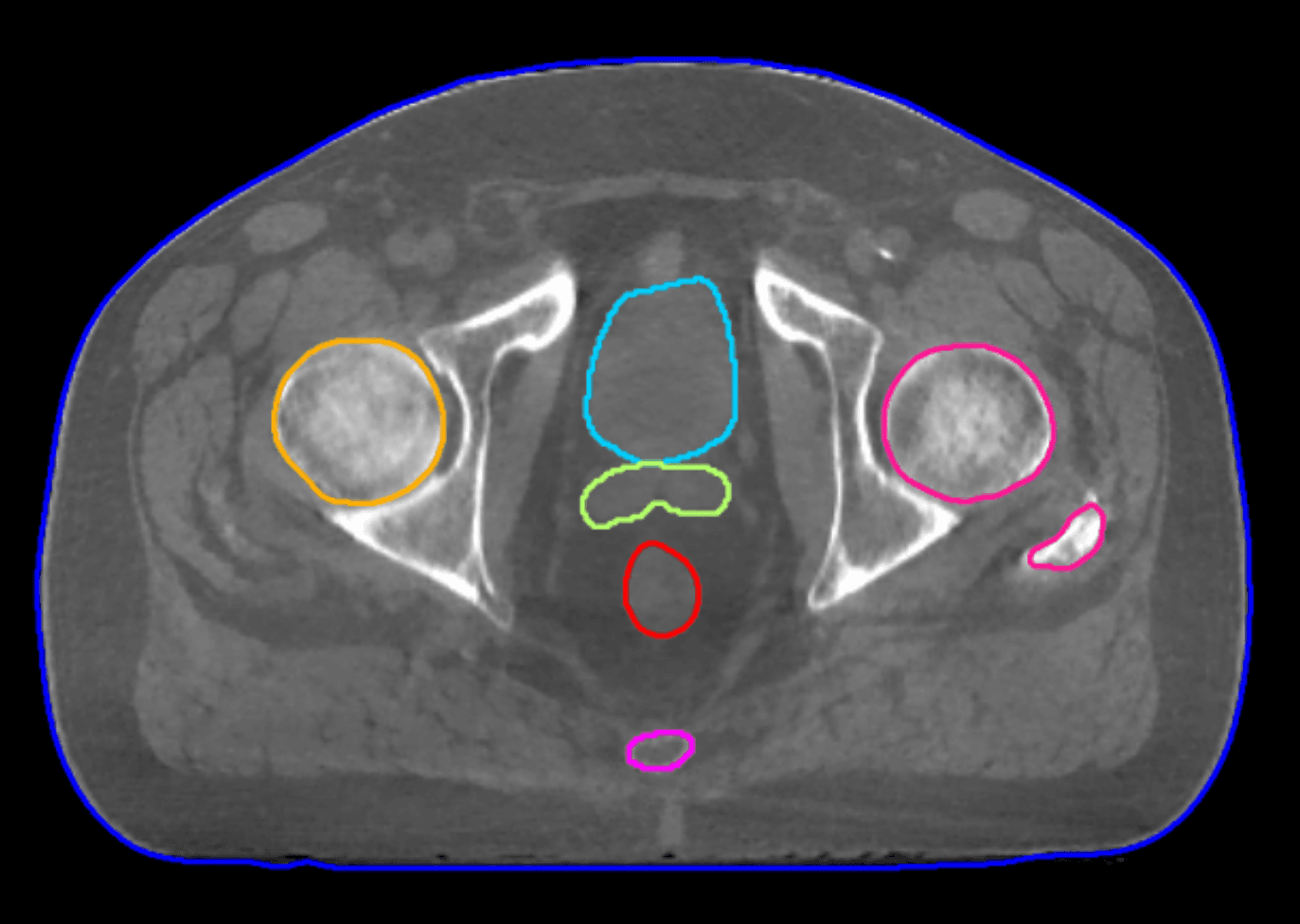
CBCT images are contaminated by high levels of scatter radiation, which are all the X-rays deflected by the patient or linac. Scatter can degrade image quality by introducing shading artifacts and reducing contrast, necessitating compensation during reconstruction.
“The problem of scatter and its resulting artifacts in CBCT imaging has been something that clinicians have had to deal with for quite some time, and they tell us this frequently,” Dr. Mason says. “We appreciate their concerns and have always strived to find solutions for this issue. These latest developments directly address this customer pain point.”
“We gave the model tens of thousands of examples and over time it learns the mapping between the primary beam being attenuated by the patient and the scatter.”
In Elekta’s Iris*, the core is an AI scatter correction model, where scatter is estimated in real-time during CBCT acquisition.
“We trained an AI model to be a scatter predictor, such that – given an X-ray image – we ask the AI model what the corresponding scatter would look like associated with that image,” he says. “We gave the model tens of thousands of examples and over time it learns the mapping between the measured X-ray frame and the scatter. When we give it a novel X-ray image the model can very accurately predict the scatter in that image.
“In essence, we’ve completely parallelized the scatter estimation task during acquisition,” Dr. Mason adds. “This means that any time overhead we’re going to have on forming an image in the viewer after acquisition is just on the 3D reconstruction.”
Reconstruction algorithm
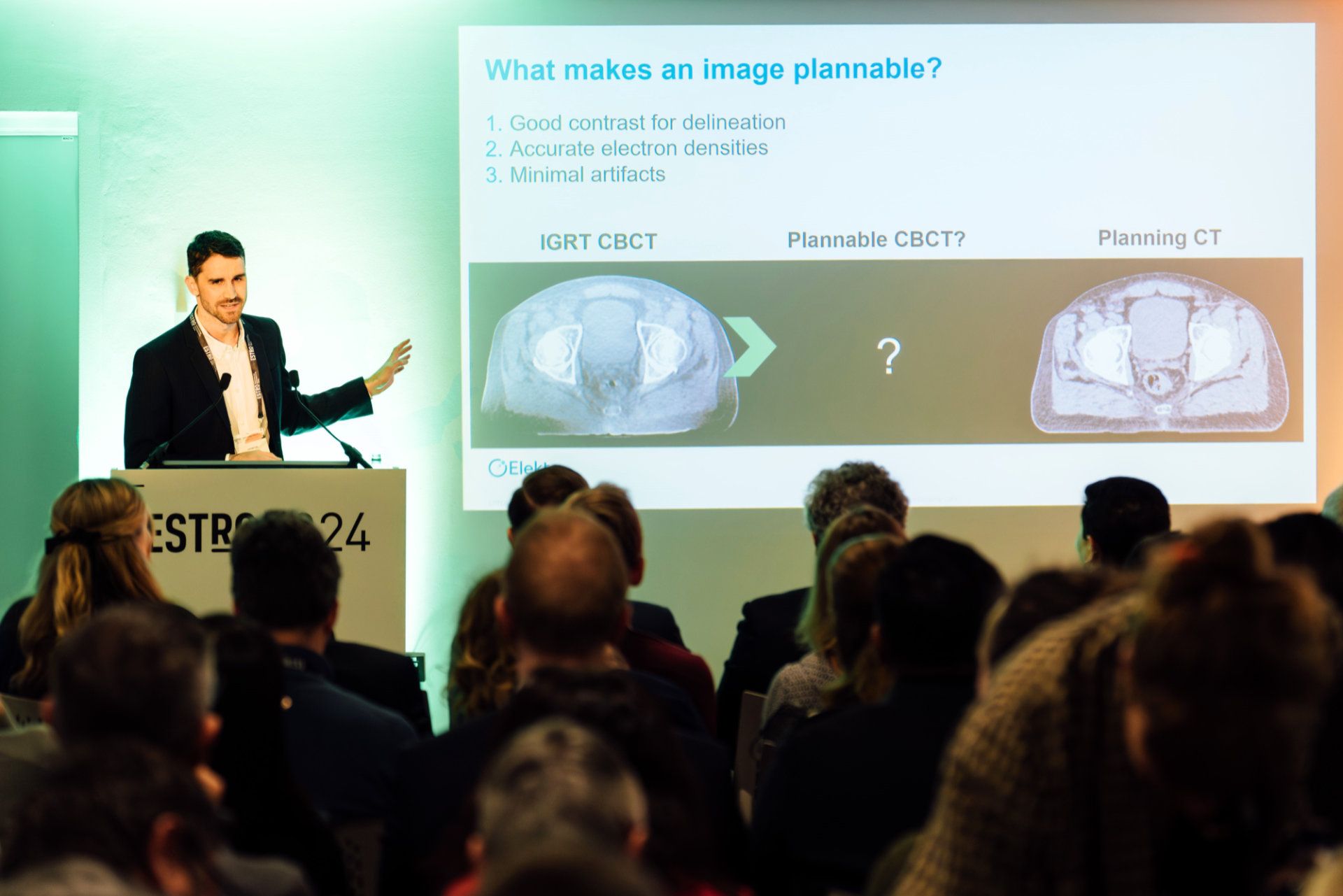
The reconstruction algorithm – polyquant CT (polychromatic + quantitative) reconstruction – is used in CT to give a significantly more accurate image with fewer artifacts.
“CBCT reconstructions are conventionally too poor to be used as the basis for planning, however, due to their insufficient sampling, beam hardening and high scatter level,” he explains. “In my PhD work, which I helped further develop at Elekta, we created an iterative polyquant method to overcome these limitations. Through this physics-driven model we get more accurate images and enhanced contrast, which is more like CT planning contrast, and provide flexible quantitative imaging for direct dose calculation.”
Putting it together for a fast adaptive workflow
With AI-estimated scatter, combined with polyquant CT reconstruction and GPU-based computation, Elekta’s engineering teams have succeeded in implementing this solution – from acquisition into a viewer – in less than 10 seconds.
“We have had several clinical research partners test the new image quality… and the feedback has been really positive.”
“We think we even have the scope to go much lower than 10 seconds,” Dr. Mason predicts. “We have had several clinical research partners test the new image quality for tasks such as HU similarity to planning CT, AI auto-contouring, registration and direct dose calculation, and the feedback has been really positive. They have suggested that Iris not only unlocks adaptive planning but enhances their existing clinical workflows.
*Iris is a component within a medical device, is not yet commercially available and requires regulatory registrations.
LAROX240712