Improving daily treatment with proton arc therapy
Dr. Leo Ding shares his experience and thoughts on the therapy and its promise for the future

Proton arc therapy is an advanced radiation therapy technique that consists of a continuous delivery of a proton beam as the gantry rotates around the patient. During this rotation, the beam energy and intensity are adjusted to confer higher conformality to the tumor, more efficient delivery and increased plan robustness. Preliminary results for Spot-scanning proton arc therapy (SPArc) have demonstrated potential clinical benefits for various disease sites, including prostate, head-and-neck, lung and brain cancers. Elekta spoke with Xuanfeng (Leo) Ding, PhD, Lead Medical Physicist, Proton Beam Therapy at William Beaumont Health System (Royal Oak, Michigan, USA) about his experience and thoughts about proton arc therapy and its promise for the future.
Elekta: How have you seen proton therapy evolving in the last few years?
Dr. Ding: From the technology side in past decade, there has been a dramatic change in the field of proton beam therapy. We are moving from passive-scattering to pencil-beam scanning (PBS), and we’re now developing rotational proton arc therapy. It is just like the era of linac development in the 1990s to 2000s, in which CBCT, IMRT and VMAT were invented and clinically implemented. That changed the entire landscape of photon radiation therapy. Similarly, we are now in the middle of the revolutionary development of proton beam therapy.
Elekta: What are the important trends in proton beam therapy?
Dr. Ding: In my view there are two key trends. First, is the continuous development of treating and imaging technology. New innovations in these areas aim to provide more robust, fast and accurate patient treatments. Second is the ongoing reduction in investment and operational costs with the proton system – so more proton centers can be introduced into local communities and more patients can access these advanced cancer treatment technologies within a reasonable driving distance.
“Arc therapy solves three major challenges: those associated with dose conformity, treatment efficiency and the need for a more robust treatment dose.”
Elekta: Why is arc therapy, specifically, important to implement for protons?
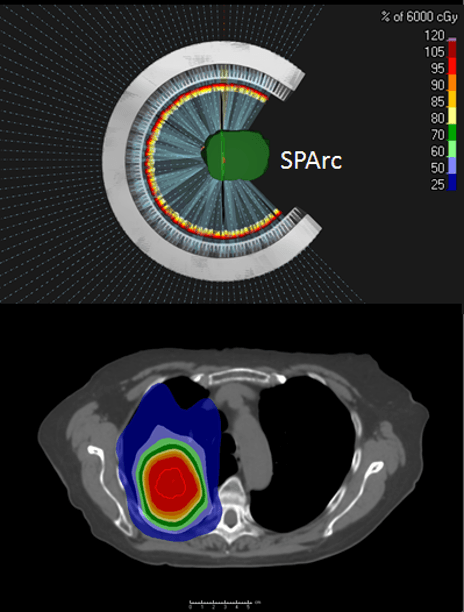
Dr. Ding: Arc therapy solves three major challenges: those associated with dose conformity, treatment efficiency and the need for a more robust treatment dose.
Regarding dose conformity, the current IMPT technique uses only a limited number of treatment fields, which may not provide optimal treatment plan quality. This could be solved by delivering more beam angles, but delivery efficiency is slow using protons, and proton centers can’t afford that beam time. So, the proton arc opens up the degree of freedom to the arc trajectory to optimize a better and faster treatment plan.
Considering treatment efficiency, arc therapy simplifies the clinical treatment workflow compared to conventional IMPT, which effectively improves the throughput of a proton center. It could increase the revenue of the proton center and more patients could be treated in each proton beam therapy facility.
Lastly, arc therapy provides a more robust treatment dose against uncertainties, which was the weakness of conventional proton beam therapy. More recently, we published a series of papers on lung cancer SBRT and spine mets SBRT that showed tremendous improvement in robustness.
Elekta: Why has this approach not emerged earlier?
Dr. Ding: There are three reasons, the first of which is technology limitations. A proton gantry weighs hundreds of tons. To deliver a proton spot with submillimeter accuracy while rotating such a giant machine is a big engineering challenge.
Next is the energy layer switching system. The technique was not matured at that time, which cost several seconds to switch each energy layer. Consequently, to deliver an arc plan with hundreds of energy layers 10 years ago would have taken 30 minutes or more. It was just not feasible to implement it in clinical routine, so there was no market at that time.
The third issue was that there was no single treatment planning system (TPS) on the market that could generate such complicated proton arc plans. As a result, no one had demonstrated that such a concept was compatible with the existing clinical proton system, and no one had conducted a series of comprehensive studies to demonstrate the potential clinical benefits utilizing proton arc therapy.
About six years ago, our team – Dr. Li and I were still very young and naïve at that time – believed in this concept. So, we worked day and night to solve this problem, and the result is promising and exciting. Our proton vendor, IBA, believed in us and collaborated with us on this ambitious project. And now, it works. We delivered the first prototype proton arc therapy using a clinical system at William Beaumont in 2018. This is one of the milestones that changed peoples’ minds that arc therapy is not a concept anymore. It has become reality.
Elekta: Intuitively, proton arc seems to be linked to an increased dose bath. Doesn’t this conflict with what proton therapy is trying to achieve?
Dr. Ding: Yes and no – it depends on how you define the dose bath. Compared to the conventional IMPT, we found that arc therapy can reduce about ten percent of the patient’s body integral dose in different disease sites because of its better conformity, and because it’s optimized for a shorter beam path. A low dose bath – 0.5 Gy or 1 Gy volume, for example – will be higher than IMPT. Such a low dose bath could be very important to our pediatric patient population, in which the chance of secondary malignancy may increase. Still, proton arc is much better that VMAT. In these clinical situations, clinical users can use partial arc therapy instead of a full arc. Therefore, it offers an equivalent dose distribution compared to IMPT. Note that I am not saying arc therapy is going to replace everything. This is just a new treatment platform; the user can use it wisely to benefit patients who need this technology.
Elekta: How exactly does proton arc therapy improves the robustness of a plan?
Dr. Ding: With the degree of freedom through the arc trajectory. We demonstrated the improved plan robustness in multiple disease sites through a series of publications, lung mobile treatment, for instance, which mitigates the breathing-induced motion interplay effect and spine mets SBRT, for example, which showed that proton arc can mitigate dose perturbation for situations in which the patient geometry changes. So, degree of freedom matters a lot in proton beam therapy. People may not like the low dose bath, but it helps in treatment robustness.
Elekta: And how does it impact patient throughput?
Dr. Ding: There are three mechanisms for this, the first of which is that it reduces the number of isocenters. If the patient’s tumor or target is large, such as the chest wall or breast – which requires two isocenters to cover the entire target – SPArc can irradiate the entire target through one isocenter. Saving one isocenter reduces by about five minutes the time it takes for couch movement and the imaging validation process.
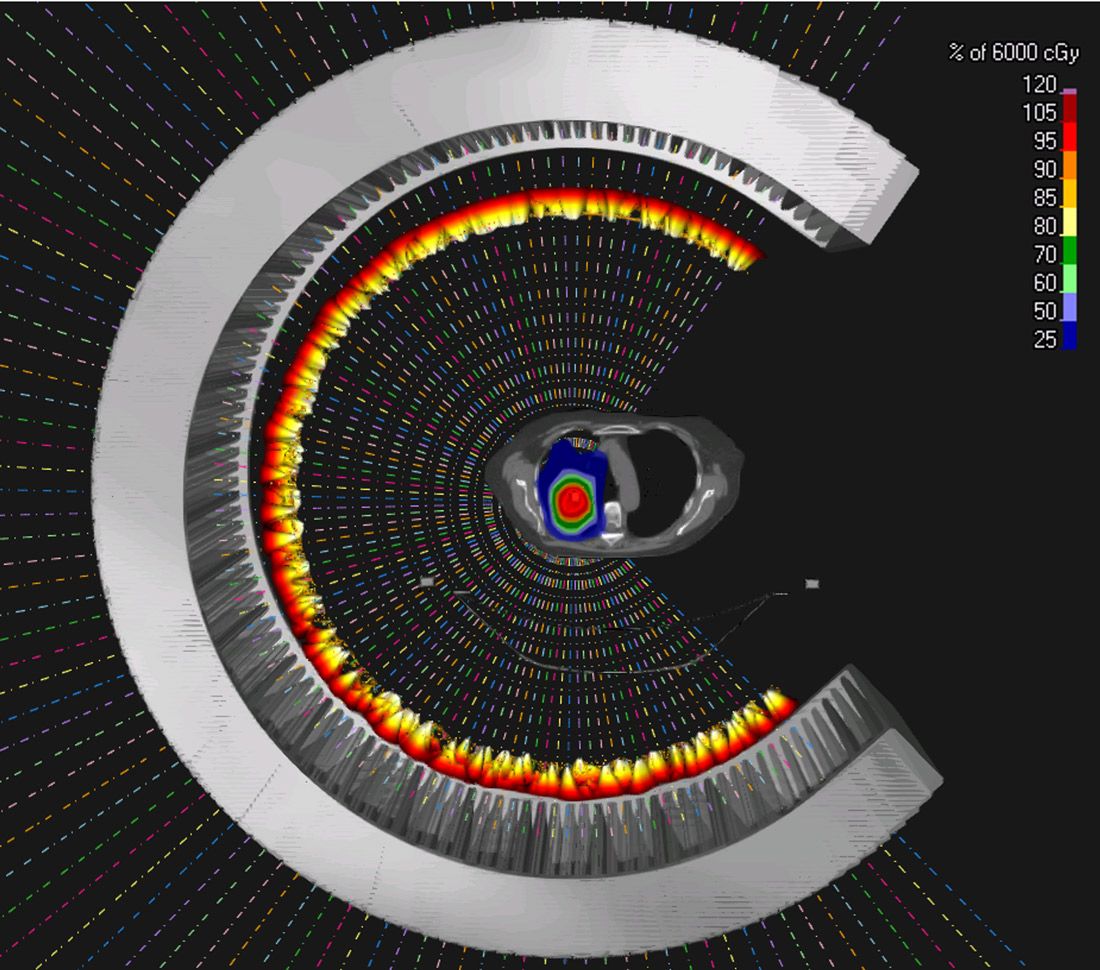
Secondly, arc therapy simplifies the treatment workflow. Normally, therapists need to rotate the gantry to the exact angle and then request the proton beam. With proton arc, our therapists only need to request it once. Treatment delivery is automatic, and the gantry rotates automatically. This simplification saves a lot of time in the clinic. Based on statistics from our own proton center, an IBA single-room system, we are expected to treat 20 to 25 percent more patients per day. I think it would work even better for a multi-room system.
Lastly, treatment of mobile tumors using a repainting technique may take a lot of time using IMPT SBRT. SPArc therapy could provide the same level of robust target coverage as volumetric repainting, about which we published this year on lung SBRT.
“We did find a lot of potential clinical benefits for a broader range of clinical indications, such as non-small cell lung cancer, head-and-neck cancers, brain tumors, prostate cancer and others. All of the dosimetric plan qualities are significantly improved compared to conventional IMPT.”
Elekta: What are the best indications for proton arc therapy and why?
Dr. Ding: It’s difficult to provide a definitive answer today. We are still in the preliminary investigation stage. However, based on the current studies that we published, we did find a lot of potential clinical benefits for a broader range of clinical indications, such as non-small cell lung cancer, head-and-neck cancers, brain tumors, prostate cancer and others. All of the dosimetric plan qualities are significantly improved compared to conventional IMPT.
Whether or not such dosimetric quality improvement will translate into better clinical outcome, we don’t know at this moment. But I am very confident it will do so, just like IMRT did to 3D conformal. So, let’s first make arc therapy clinically available to our patients.
Importantly, our radiation oncology society is moving toward the hypofractionated treatment. Conventional IMPT was not widely considered as an SBRT/SRS treatment option for many reasons. For example, inferior dose conformity and more sensitivity to uncertainties. But I think the SPArc technique will change this paradigm. It could become a new standard treatment option for SBRT/SRS, especially in the era of concurrent chemo-RT or immuno-RT. This new technology will help the proton market expand its clinical indications and benefit more patients in a hypofractionation regimen.
“I am glad to see Monaco now entering the proton world with an FDA-cleared clinical solution.”
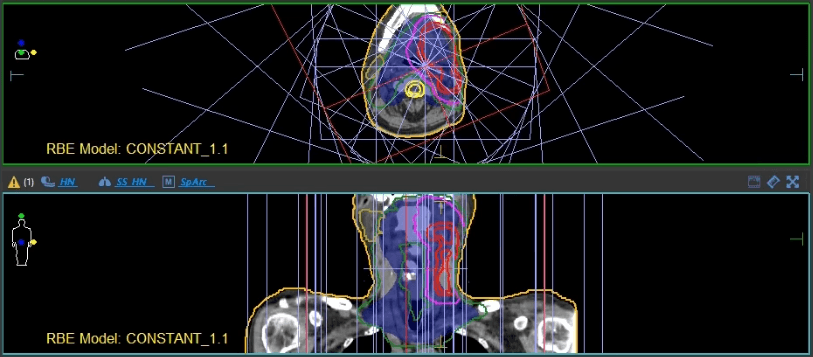
Elekta: How can Elekta help you bring this new technology to the market?
Dr. Ding: Elekta can definitely help. There are two main ongoing aspects of development. First is the proton beam therapy system itself, which allows us to dynamically rotate the gantry while delivering the proton radiation. In this direction, we are collaborating with IBA, our proton manufacturer. Second, is the TPS to support this advanced treatment modality. TPS has played a key role in radiation therapy for decades. Without a TPS vendor, there is no way we could implement such a nice technique into routine clinical practice. I’ve used Elekta’s Monaco® TPS since I was a graduate student in photon radiotherapy 15 years ago. And I am glad to see Monaco now entering the proton world with an FDA-cleared clinical solution. With all the expertise and developers, the collaboration between Elekta and our clinical team will lead to a better, more efficient proton arc optimization platform, which is critical to faster adoption of this new technology in our community for the benefit of more cancer patients.