Adaptive radiotherapy poised to transform traditional linac treatments

Improvements in CBCT image quality will soon make adaptive RT workflow routine for many clinics
Just as pristine MR image quality enabled clinicians to confidently implement online adaptive radiotherapy (ART) using the MR-Linac system, technological innovations in cone beam computed tomography (CBCT) image quality and the reconstruction algorithm will drive the use of offline and online ART among users of suitably equipped conventional CT-Linacs. Elekta has been refining this technology for decades and its introduction to the industry is imminent, according to John Christodouleas, MD, Elekta’s Senior Vice President of Medical Affairs and Clinical Research.

“Over the last 40 years, the field of radiation oncology has been steadily bringing the advanced imaging technologies from the diagnostic world and integrating them with a linear accelerator. For example, Elekta integrated high-field MRI with the treatment system in the Elekta Unity,” Dr. Christodouleas says. “We are doing something similar with CT imaging. The imaging is getting better and better, and it has to. CT images have to be high quality to use them as a patient model for ART. Elekta’s latest efforts have borne fruit and that will be reflected in new technology offerings this year.”
ART key to treating a dynamic system like the human body
Exceptional image quality is essential in diagnosing many pathologies in the human body and equally important in radiation therapy. Radiotherapy imaging has evolved from 2D radiation therapy to CT-simulation, to 3D CT volume imaging – a snapshot in time that would be used for a patient’s entire treatment course. The latest development, ART – whether it’s offline or online replanning – accounts for the dynamic nature of the human body.
“The body is constantly changing – tumors are shrinking, normal tissues are moving around, bladders and bowels are full some days and empty on other days,” he says. “Adaptive is simply updating the patient model and you can do that every once in a while, once or twice a week during a therapy course, or you could do it every day right before you treat. In the case of MR-guided radiation therapy, clinicians routinely update the patient model every day. Eventually they will be able to replan while the beam is delivering dose.”
The goal of ART is to maximize the therapeutic dose to the target, while minimizing the dose to adjacent normal tissues. While IMRT has been useful in shaping the high dose bath more precisely around the tumor, ART is the next step in radiotherapy precision, according to Dr. Christodouleas.
“The task of radiotherapy for the last four decades has been generating ever more confidence to reduce those safety margins that cause us to treat normal tissues unnecessarily. ART will be instrumental in realizing this goal.”

“IMRT doesn’t account for time and the changes that occur with time, which are random and systematic variations in the target’s shape and position,” he observes. “You have to compensate for the expected change in shape and position of the target [i.e., PTV]. The task of radiotherapy for the last four decades has been generating ever more confidence to reduce those safety margins that cause us to treat normal tissues unnecessarily. ART will be instrumental in realizing this goal.” (see side bar)
Certain indications, such as a head-and-neck cancer, present systematic variations because the target’s size and shape, rather than its position, are generally the only factors to consider as the target is stationary during treatment.
“A head-and-neck cancer typically shrinks slowly over time,” Dr. Christodouleas says. “You don’t need to necessarily adapt the plan every day or do it online – most centers adapt once or twice a week.”
Conversely, a cervical cancer tumor is subject to random variations in shape and position.
“The target’s position and shape are different for every fraction because they are defined by bladder filling, which is random – a person’s bladder is full or less full, depending on a number of factors, so you can’t perfectly control it,” he says. “When the clinician creates a traditional PTV, it’s the union of several potential shapes, because you have to cover every position of the target. To optimally account for these random interfraction variations you have to do online daily adaptation to compensate for the bladder filling for that day.”
Imaging as an ART enabler
Elekta’s efforts in developing CT-adaptive radiation therapy have centered around improving the software that determines the quality of imaging.
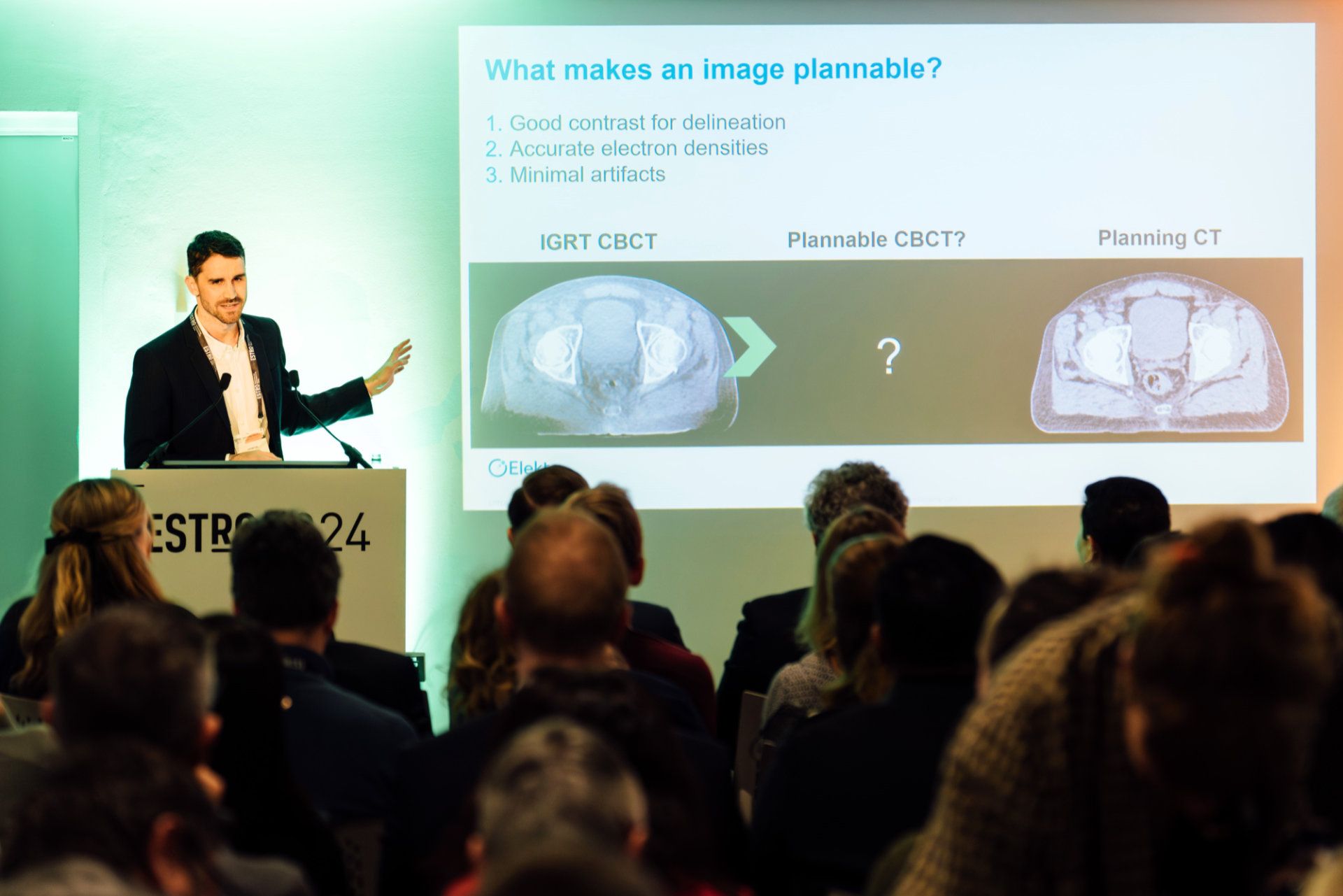
“In Elekta’s new approach to CBCT acquisition improves two key components of the process: the scatter prediction model and the underlying iterative reconstruction model. The resulting technology dramatically improves image quality and saves time.”
“Generating a CT or CBCT image is a complicated process that includes many steps,” Dr. Christodouleas says. “Elekta’s new approach to CBCT acquisition improves two key components of the process – the scatter prediction model and the underlying iterative reconstruction model. The resulting technology dramatically improves image quality and saves time.”

During the process of CT or CBCT imaging, x-rays can be deflected from their original path. This x-ray scatter creates noise and artifacts and reduces image contrast. Elekta’s new algorithms use AI techniques to very rapidly predict and reduce the negative effects of scatter on the image.
“The new scatter prediction models work on the timescale of milliseconds,” he explains. “The other big change we are making is to replace the underlying model of the reconstruction algorithm. The new model is called “polyquant” which is a combination of the words ’polychromatic‘ and ’quantitative.’ This model accounts for the fact that the beam used for imaging is made up of many energies [polychromatic]. In fact, each energy is modeled separately. When this is done, the result is a much better prediction.”
With the introduction of CT-adaptive linacs, many of the indications that clinicians are treating using MR-guided radiation therapy will become targets for CT-guided radiotherapy, according to Dr. Christodouleas.
“For example, if you’re doing daily adaptive for bladder cancer, you can see the whole bladder on CT, so it’s a perfect indication,” he observes. “Similarly, if you’re using ART for pelvic lymph node treatments, you can see them with CT, and the structure you’re adapting to is the bowel around the lymph node. So, if you can see the bowel, you can adapt to a different position in the bowel. That’s another great target. Clearly, there are some targets you won’t adapt using a CT-Linac, because you can’t see them with CT, like an evolving liver cancer, for instance. That’s why our world is going to be both CT and MR adaptive.”
A new era of adaptive radiotherapy
In addition to CBCT imaging and reconstruction enhancements, automation is improving and auto-segmentation, image registration, QA, motion management and plan re-optimization are accelerating to the point that it will simply be easier to adapt treatment than to not adapt, according to Dr. Christodouleas.
“Elekta is all-in on maximizing diagnostic quality imaging at the device and we are all-in on adaptive.”
“In fact, in the coming years, most of us will view ‘the way we used to treat’ – using a patient model that is sometimes eight weeks old – as utterly primitive,” he says. “Elekta is all-in on maximizing diagnostic quality imaging at the device and we are all-in on adaptive. That’s our strategy and we are happy to sing it from the rooftops. We are going to give our users the best imaging on the device, both CT and MR, and a toolkit for adaptive. Clinicians will be able to adapt easily whenever they think it can help their patients.”
Small PTV reductions = big reductions in normal tissue irradiation
Although ART enables seemingly nominal reductions (e.g., 5 mm) in the margin around a tumor or post-surgical cavity, the result can be significant in terms of normal tissue irradiation.
Figure 1 shows a post-prostatectomy volume with a typical (i.e., non-ART) PTV of one centimeter circumferential and 0.6 centimeters posterior for a target volume of 132 cc’s.

“When you make an expansion to account for uncertainties, you actually end up treating 348 cc’s of tissue with the biologically active high dose bath,” Dr. Christodouleas explains. “That means most of the volume that you’re treating – 216 cc’s – is actually normal tissue that is not at risk for harboring cancer. It’s simply being targeted in space because you don’t know where this tissue is going to be on any given day.
“Imagine if you had an approach that could completely eliminate this PTV – we refer to that as PTV-free radiation therapy,” he continues. “That 216 cc’s of tissue wouldn’t get the high dose bath that could only cause side effects – it can’t treat the cancer at all. Elekta’s vision for all treatments is zero PTVs. If we can get the uncertainties around delivery to less than the penumbra, then we’ve achieved PT-free radiotherapy. That’s a doable goal and what we are trying to achieve with ART.”
Learn more about Elekta’s portfolio of linear accelerators.
LAROX240917
*Future Elekta linac solutions may require market access registrations prior to commercial availability.